Coronavirus disease 2019 (COVID-19)
Authors: Dr Greg Brogan, Dr Neil Campbell, Dr Matthew Durie, and Dr Chris Nickson from the Alfred ICU (@INTENSIVEblog)
First published 6 April 2020; reviewed and revised 19 June 2020; updates since 30 May 2020 include:
- Clear reference to National COVID-19 Clinical Evidence Taskforce guidelines
- Updated evidence on remdesivir, hydroxychloroquine and convalescent plasma
- Stratified novel/experimental treatments by probability of benefit
- Moved remdesivir to ‘specific treatments’ given ACTT-1 but also kept in experimental section given uncertainty for critically ill patients
- Cut down experimental anticoagulation section
- Included recommendation for increased dose VTE prophylaxis
- Changed NIV section to be in line with current Australian guidelines
Editor’s note: This document continues to be reviewed monthly given our rapidly evolving understanding of the assessment and management of COVID-19 from a critical care perspective.
OVERVIEW
The unfolding COVID-19 pandemic has led to a global crisis which threatens to become a health, economic and humanitarian disaster
- SARS-CoV-2 is a betacoronavirus first described following a case cluster of patients with pneumonia of unknown cause in the Wuhan province in China (Zhu N. et al. 2020).
- “COVID-19” or COronaVIrus Disease 2019 is the term used by the WHO to refer to disease caused by this virus. The virus was also called 2019-nCoV (or 2019 novel CoronaVirus) prior to being official named by the WHO. [Coronaviridae Study Group. 2020]
- COVID-19 is predominantly a respiratory disease, with severity ranging from mild to fatal, and transmission mostly from the spread of respiratory droplets.
Pandemic timeline:
- Disease first identified in Wuhan City, Hubei Province, China in November 2019
- WHO declared a global emergency on 30 January 2020
- WHO declared a global pandemic on 11 March 2020
CAUSE AND RISK FACTORS
Virology
- SARS-CoV-2 is the seventh strain of coronavirus known to infect humans (Coronaviridae Study Group. 2020; Chen Y. et al. 2020). Coronaviruses are named for their characteristic halo when visualised by electron microscopy. Four strains circulate freely among humans (HCoV-229E, HCoV-OC43, HCoV-NL63 and HKU1), while many others infect animal hosts (Chen Y. et al. 2020).
- In addition to the four endemic strains, two others have demonstrated animal-to-human transmission and resulted in documented outbreaks (Chen Y. et al. 2020):
- Severe Acute Respiratory Syndrome (SARS-CoV) in 2002
- Middle East Respiratory Syndrome (MERS-CoV) in 2012
- SARS-CoV-2 probably has a zoonotic origin.
- SARS-CoV-2 shares 96% genetic similarity to a bat coronavirus (Zhou P. et al. 2020), although the source species is not known with certainty.
- Some household animals, including dogs, can be infected with SARS-CoV-2, but they are not known to have a role in transmission to humans (Sit et al, 2020).
Transmission
- SARS-CoV-2 is transmitted person-to-person, predominantly by respiratory droplet spread and contact, similar to the MERS and SARS coronaviruses (Otter J. et al. 2015, Lai C. et al. 2020)
- Larger respiratory droplets (>5 μm) remain in the air for only a short time (<17 minutes) and travel only short distances, generally <1 m (Kutter et al, 2018).
- Respiratory droplets can probably transmit > 1 m in certain circumstances, such as being driven by airflow from air conditioned ventilation (Lu et al, 2020) or by sneezing (Bourouiba, 2016).
- The dichotomy between “airborne” and “droplet” precautions is based on arbitrary droplet size cutoffs may not accurately reflect what actually occurs with respiratory emission (Bourouiba, 2020).
- SARS-CoV-2 has a higher level of shedding from the upper respiratory tract than SARS-CoV-1, even in asymptomatic/ presymptomatic cases, which may have contributed to its wider spread (Gandhi, M. et al 2020)
- Contact transmission
- The SARS-CoV-2 virus may survive for up to 4 days when respiratory droplets settle on surfaces, based on in vitro experiments (van Doremalen N. et al. 2020).
- Transmission occurs when contact with virus-laden objects (fomites) is followed by contact with mucous membranes (e.g. eye, nose, mouth) from face touching.
- Aerosol transmission
- Aerosol transmission of SARS-CoV-2 virus has not been demonstrated, but remains a possibility (Morawska, 2020).
- Aerosolised coronavirus (non-SARS-CoV-2) particles ≤ 5µm have been demonstrated from infected subjects (Leung N. et al. 2020) and some studies found transmission that could be explained by “opportunistic” airborne spread (Roy et al, 2004)
- The SARS-CoV-2 virus may be viable for at least 3 hours in aerosolised environments, based on in vitro experiments (van Doremalen N. et al. 2020).
- The effectiveness of airborne precautions for respiratory viruses, compared with droplet precautions, is controversial (Shiu et al, 2019),
- SARS-CoV-2 RNA has been detected from ambient air samples in patient isolation rooms (Zhen-Dong G. et al. 2020).
Other routes
- The SARS-CoV-2 virus may be shed in stool, raising the unconfirmed possibility of fecal-oral transfer (Yeo C. et al. 2020). In some cases this may persist beyond the duration of respiratory viral shedding (Tang A. et al. 2020).
- Vertical transmission (mother to child) rates appear low (as with SARS-CoV) (Zhu H. et al. 2020)
Figure 1. Summary of viral structure, life cycle, clinical course, and immune response for COVID-19. Image shared by Abby Schiff, PhD (@liraphila). Image source: https://twitter.com/liraphila/status/1250871681591267328. Source of original work: https://www.cell.com/pb-assets/products/coronavirus/CELL_11362_S5.pdf
PATHOGENESIS AND DISEASE PROGRESSION
Pathogenesis
- Coronaviruses express trans-membrane glycoproteins (“spike” proteins) which allow the virus to attach to and gain entry to the target cell.
- Spike proteins on SARS-CoV-2 share many similarities with those of SARS-CoV and bind to surface Angiotensin Converting Enzyme 2 (ACE2) receptors (Walls A. et al. 2020; Zhang C. et al. 2020).
- ACE2 is expressed predominantly on type II pneumocytes but also on upper respiratory tract epithelial cells and small intestine enterocytes (Hamming I. et al. 2004).
- The SARS-CoV-2 spike protein appears to bind ACE2 with higher affinity than SARS-CoV, which may account for its greater transmissibility (Wrapp D.et al. 2020)
- Other cofactors are likely required, including TMPRSS2 (Hoffman M. et al. 2020)
- Viral RNA replication occurs within the target cell, utilising RNA-dependent RNA polymerase (rdRp) (Lung J. et al. 2020).
Incubation period
- Median incubation period is 4-5 days
- symptoms develop:
- between 2 and 7 days in 75% of cases (Guan W. et al. 2020)
- after 14 days in less than 1% of cases (Lauer S. et al. 2020)
Disease progression
- Asymptomatic viral shedding may occur at some stage in up to 50% of patients, although this remains contentious (see below)
- Most (80%) COVID-19 cases experience mild-to-moderate URTI symptoms for the first 7 days followed by recovery (Bouadama L. et al. 2020).
- Approximately 20% of patients develop dyspnoea requiring hospital admission, typically at day 6-8.
- A small proportion of patients with SARS-CoV-2 infection develop life threatening lung disease characterised by severe pneumonitis that may progress to acute respiratory distress syndrome (ARDS)
- Involves diffuse, direct and indirect damage to alveoli
- profound hypoxaemia with relatively preserved lung compliance appears common early
- Some severely affected patients also develop:
- “Cytokine storm” – a severe reaction akin to hemophagocytic lymphohistiocytosis (HLH) (Ruan Q. et al. 2020)
- Myocarditis/ cardiomyopathy (rare) (Arentz M. et al. 2020)
- Disseminated intravascular coagulation, which portends a poor prognosis (Tang et al. 2020), and thrombotic complications
- Post-mortem findings include: (Menter T. et al. 2020, Wichmann D. et al. 2020)
- Diffuse alveolar damage typical for ARDS
- Lymphocytic infiltration
- Microvascular and large vessel thrombosis
- In some cases, a secondary bacterial pneumonia with or without underlying diffuse alveolar damage
- Extrapulmonary features including lymphocytic myocarditis
- Presence of SARS-CoV-2 RNA in lung and other tissues
Spectrum of severity
SARS-CoV-2 infection may result in illness ranging in severity from no symptoms, to mild, moderate or severe COVID-19.
- Asymptomatic
- a small proportion may remain asymptomatic though the exact proportion unknown (see below)
- The proportion of severity experienced in one large cohort of patients was (Wu Z. & McGoogan J. 2020):
- Mild or moderate – 81%
- Severe – 14 % (dyspnoea, RR ≥30, oxygen saturation ≤93%, PF ratio <300, and/or lung infiltrates)
- Critical – 5% (respiratory failure, septic shock and/or multi organ dysfunction)
Severity classification according to Australian guidelines for the clinical care of people with COVID-19 (v2):
| Mild Illness | Person not presenting any clinical features suggesting a complicated course of illness. Characteristics: – no symptoms or mild upper respiratory tract symptoms – stable clinical picture |
| Moderate Illness | Stable patient presenting with respiratory and/or systemic symptoms or signs. Able to maintain oxygen saturation above 92% (or above 90% for patients with chronic lung disease) with up to 4L/min oxygen via nasal prongs. Characteristics: – prostration, severe asthenia, fever > 38 ̊C or persistent cough – clinical or radiological signs of lung involvement – no clinical or laboratory indicators of clinical severity or respiratory impairment |
| Severe Illness | Patients meeting any of the following criteria: – respiratory rate ≥ 30 breaths/min – oxygen saturation ≤ 92% at a rest state – arterial partial pressure of oxygen (PaO2)/ inspired oxygen fraction (FiO2) ≤ 300 |
| Critical Illness | Patient meeting any of the following criteria: – Respiratory Failure – Occurrence of severe respiratory failure (PaO2/FiO2 ratio < 200), respiratory distress or acute respiratory distress syndrome (ARDS). This includes: – patients deteriorating despite advanced forms of respiratory support (NIV, HFNO) OR – patients requiring mechanical ventilation OR – other signs of significant deterioration, hypotension or shock, impairment of consciousness, or other organ failure |
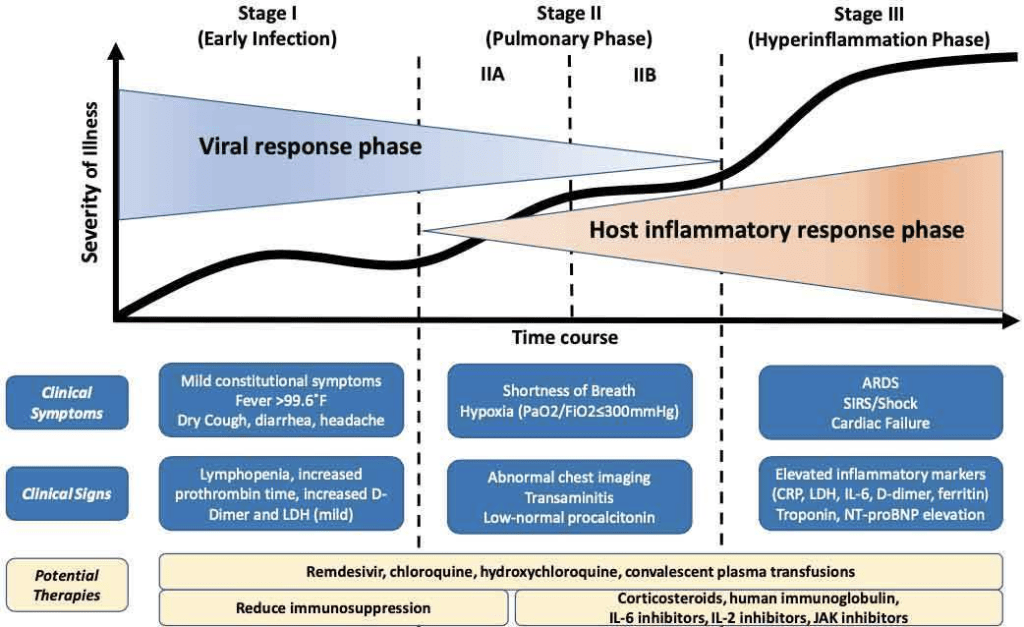
CLINICAL PRESENTATION
Common symptoms
Common presenting features include (Bouadama L. et al. 2020, Young B. et al. 2020):
- Fever
- Incidence varies depending on study (~40-90%)
- Tends to be high and persistent
- Cough
- Breathlessness
- Dyspnoea onset tends to be around Day 6
- Multiple reports, especially in elderly, of ‘silent hypoxia’ – severe hypoxaemia without breathlessness
- Anosmia
- Olfactory and/or taste disturbance in approximately one third of patients (Giacomelli A. et al. 2020)
- In South Korea, 30% of those testing positive had anosmia as their major presenting symptom in otherwise mild cases (Iacobucci G. 2020)
- Less commonly or rarely
- Rhinorrhoea
- Sore throat
- Myalgia
- Gastrointestinal symptoms (e.g. diarrhoea)
- Other neurological features
- Meningitis/ encephalitis and hemorrhagic necrotizing encephalopathy (including altered mental state and coma) (Moriguchi, 2020; Poyiadji, 2020)
- Guillain-Barre Syndrome (Toscana, 2020)
- encephalopathy, agitation, confusion, and corticospinal tract signs in COVID-19 ICU patients with ARDS (Helms, 2020)
Asymptomatic infection
- In high transmission-risk environments (e.g. cruise ships, nursing homes) approximately 50% of cases experience an asymptomatic period in which viral RNA can be detected from upper respiratory tract samples (Mizumoto K. et al. 2020, Nishiura H. et al. 2020, Arons M. et al. 2020).
- True asymptomatic proportion unknown (ie. those who never develop symptoms). In two cruise ship cohorts it was estimated between 17% and 30% Mizumoto K. et al. 2020, Nishiura H. et al. 2020)
- The ability of asymptomatic cases to infect others is also unknown but may havecontributed to the wider spread of SARS-CoV-2 than SARS-CoV-1 (Gandhi, M. et al 2020)
- Some asymptomatic patients may still manifest laboratory and radiographic changes (Hu Z. et al. 2020).
COVID-19 pneumonia “phenotypes”
- A controversial report suggested that COVID-19 patients appear to have at least two phenotypes, from the perspective of ICU management (Gattinoni et al, 2020). However, this classification is largely based on anecdote, remains preliminary and management should be optimised for each individual patient as clinically indicated.
- L-phenotype
- Typical of early presentation viral pneumonitis
- Hypoxaemia with preserved CO2 clearance (Type 1 respiratory failure)
- Low
- Elastance (i.e. high compliance)
- V/Q matching (possibly due to abnormal hypoxic vasoconstriction)
- Recruitability (poor response to PEEP and proning)
- Implications
- May be able to avoid mechanical ventilation with appropriate oxygen therapy
- May be responsive to pulmonary vasodilators (e.g. inhaled nitric oxide)
- H phenotype
- Typical of later illness and classic ARDS, including patients who have had prolonged non-invasive ventilation (potential for patient-induced lung injury from volutrauma and barotrauma) and co-existing lung disease or complications
- Hypoxaemia +/- impaired CO2 clearance (Type 1 and/or 2 respiratory failure)
- High
- Elastance (i.e. low compliance)
- V/Q matching
- Recruitability ( response to PEEP and proning)
- Implications
- May benefit from protective lung ventilation and usual ARDS therapies (potentially including an “open lung approach”)
- L-phenotype
- The existence and utility of these phenotypes is being increasingly called into question as observed cohorts suggest that COVID-19 presentations are consistent with prior descriptions of ARDS (Ziehr D. et al. 2020).
DIAGNOSIS
The case definition for suspected COVID-19 has changed over time. A set of definitions is available from Communicable Disease Network Australia (CDNA v2.7, 2020), but clinicians should note that this may differ from the criteria for testing within their state or region. Testing is suggested by CDNA in suspect and enhanced testing categories.
Diagnosis is confirmed by testing via one of the methods below.
- Confirmed Case
- Positive on nucleic acid test
- Virus identified by electron microscopy
- Viral culture
- Probable Case
- A person who has not been tested
- WITH a fever of >/38 degrees OR acute respiratory infection (cough, shortness of breath, sore throat)
- AND is a household contact with a confirmed or probable case
- Suspect Case
- Meets the following Clinical AND Epidemiological criteria
- Clinical
- Fever >/38 degrees OR history of feer
- OR acute respiratory infection (as above)
- Epidemiological
- In the last 14 days prior to illness onset:
- Close contact with confirmed or probable case
- International or interstate travel
- Passengers or crew from a cruise ship
- Healthcare, aged or residential care workers and staff with direct patient contact
- People who have lived in or travelled through specifically designated areas with elevated community transmission (defined on this website)
- OR Hospitalised patients where no other focus or alternate explanation for illness is evident
- In the last 14 days prior to illness onset:
- Enhanced testing
- It is important to check relevant state and territory case definitions as they vary of location and time, however, the Australian national recommendations as of 24/4/2020 include:
- Testing beyond the suspect case definition in patients with:
- Fever >/38 or history of same
- OR acute respiratory infection where no other clinical focus or alternate explanation is evident.
- Testing beyond the suspect case definition in patients with:
- It is important to check relevant state and territory case definitions as they vary of location and time, however, the Australian national recommendations as of 24/4/2020 include:
INVESTIGATIONS
Diagnostic tests
Viral PCR
- Real time Reverse Transcriptase Polymerase Chain Reaction (RT PCR) test of respiratory samples is the test of choice
- Nasopharyngeal or oropharyngeal samples recommended by CDC and CDNA (CDC, 2020)(CDNA, 2020)
- Samples taken from both oropharynx and nasopharynx to optimise virus detection
- Multiple PCR targets are available and improved tests are under development: (Corman, VM 2020)
- Sensitivities range from 60-85% in respiratory specimens (Chan, JFW et al 2020)
- Highly specific, some approaching 100% (Chan, JFW et al 2020)
- Sensitivity varies with specimen origin (Wang et al. 2020):
- Bronchoalveolar lavage shows highest positive rates (93%), but diagnostic bronchoscopy should be avoided where possible due to risk of aerosolization.
- Positive rates of other specimens includesputum (72%), nasal swabs (63%), fiberscopic brush biopsy (46%), pharyngeal swabs (32%), faecal (29%) and urine (0%).
- Viral shedding/positive rate may vary between both cases and throughout the course of disease (Farkas J et al 2020) – hence a single negative test does not exclude infection
Serological tests
- Acute and convalescent testing of sera to identify presence of IgM or IgG antibodies specific to SARS-CoV-2.
- Does not detect virus per se, just evidence of recent infection.
- Test will be negative until development of humoural immunity (up to two weeks, Zhao J. et al. 2020). See Immunity below.
- Limited by potential cross reactivity with other viruses.
Bedside Tests
- Point of care testing under development with viral PCR remaining the current test of choice (see above).
- These may allow a result within 15 minutes and in the field.
- May be antigen or serology based
- Antigen based – potentially allows detection of active or new infection, compared with serological tests.
- Though the sensitivity is not known, similar tests for other viruses range in sensitivity from 34-80% (WHO 8/4/2020)
- Until properly validated, the WHO recommends the use of point of care testing in a research setting only (WHO 8/4/2020)
Other
- Other methods of detection include (CDNA, 2020):
- Imaging – see Investigations below
- Viral culture – not widely recommended due to safety concerns
- Electron microscopy – may allow visualisation of virions when the viral aetiology of a disease is uncertain or when no other known tests are available. Although included by the CDNA as a method of confirming a positive case, in practice it is rarely used except in the early phases of an outbreak or for research purposes.
Screening tests
- Utility of screening asymptomatic (and pre-symptomatic) patients remains unclear (Yuen KS et al 2020).
- Antibody based screening may allow estimation of true disease prevalence and guide public health policy, however in low prevalence areas (<5%), screening tests with poor specificity may have a false positive rate higher than the true positive rate.
Diagnostic pitfalls
- False negative tests (see above)
- Co-infection with other viruses has been observed (Wu et al. 2020)
- Secondary/ co-existent bacterial infection can occur
- There is potential for hospital acquired COVID-19 to become an issue as COVID-19 becomes more prevalent among hospital inpatients.
- Unrelated co-existent conditions may be falsely attributed to COVID-19 infection if prevalence is high.
Laboratory Tests
Though many laboratory abnormalities are being described in the literature in association with COVID-19, clinicians should only order investigations that will guide ongoing management
- Viral PCR (see “diagnosis” section above)
- Full Blood Count
- Lymphocytopenia is characteristic in most studies (43%) (Rodriguez-Morales AJ et al 2020)
- In critically ill may be as high as 85% (Huang, C et al 2020)
- Lymphocytopenia is characteristic in most studies (43%) (Rodriguez-Morales AJ et al 2020)
- white cells can be low (25%), normal (45%) or high (30%), but tends to be normal/higher in critically ill
- thrombocytopenia below 100 was uncommon (approx. 5%).
- Coagulation Studies (Chen, N et al 2020)
- PT/APTT usually unchanged or slightly prolonged
- Disseminated intravascular coagulation (DIC) may occur
- Electrolytes (Guan, WJ et. al. 2020; Rodriguez-Morales AJ et al 2020)
- No characteristic electrolyte changes evident
- Creatinine usually preserved
- Blood glucose is increased in 52% (Chen, N et al 2020)
- Liver Function
- ALT/AST/Bilirubin may be elevated (Guan, WJ et. al. 2020, Rodriguez-Morales AJ et al 2020)
- LDH increased
- 40-76% of all cases (Rodriguez-Morales AJ et al 2020)
- 92% of ICU cases (Huang, C et al 2020)
- Possible marker of severity
- Acute Phase Reactants (Rodriguez-Morales AJ et al 2020)
- ESR increased (85%)
- CRP increased (86%; may be marker of severity)
- Albumin decreased (75%)
- Ferritin increased (63%; may be marker of severity)
- High ferritin (>700ng/ml) suggests risk of “cytokine storm syndrome” / HLH (Cron, R, Chatham, W 2020)
- Other laboratory tests
- Elevation of these parameters is more common in ICU COVID-19 patients:
- D-dimer
- Troponin
- Procalcitonin (not well defined, may lag behind CRP (though neither test has a clear role in management
- Lumbar puncture and CSF analysis should be considered if suspected meningitis or Guillain-Barre Syndrome
- Antiphospholipid antibodies (case reports in hypercoagulable COVID-19 patients)
- Elevation of these parameters is more common in ICU COVID-19 patients:
Imaging
- In general:
- COVID-19 findings are consistent with a viral pneumonia – i.e. there are no findings specific to CIVD-19
- COVID-19 pneumonia commonly manifests as ground glass opacities distributed bilaterally in bases and peripheries
- Findings evolve rapidly (eg. from unilateral to bilateral) and lung involvement is associated with severity; Findings progress over 1-3 weeks, hitting maximum at 10-12 days
- Findings may be present in asymptomatic individuals or pre-symptomatic individuals
- Chest x-ray
- Features Include (Guan, WJ et. al. 2020 Wong, HYF, et al. 2020):
- Bilateral shadowing (72.9%) – mostly ground glass opacity (68.5%) (Wong, HYF, et al. 2020)
- Unilateral disease (25%) (Guan, WJ et. al. 2020)
- Local patchy shadowing
- Interstitial abnormalities (less common finding, <5% in some studies) (Guan, WJ et. al. 2020)
- Pleural effusions are uncommon
- Features Include (Guan, WJ et. al. 2020 Wong, HYF, et al. 2020):
- CT imaging of the lungs
- Changes seen in 86% of cases (Guan, WJ et. al. 2020)
- Common features Include (Ye, Z et al 2020):
- Ground Glass opacities (GGOs) (98%)
- Reticular Pattern
- Consolidation
- Crazy Paving Pattern
- Uncommon manifestations seen in COVID patients (Salehi S et al. 2020, Rodriguez JCL. et al 2020)
- Pleural effusion
- Pericardial effusion
- Lymphadenopathy
- Cavitation
- CT Halo sin
- Pneumothorax
- Involved lung is proportional to severity of disease (Zhao, W et al. 2020)
- When followed over time, a pattern to stages of disease emerges (Li, M et al 2020)
- Early Phase – Moderate clinical manifestations with lesions limited to single or multiple areas
- Progressive phase – Lesions progress in extent and density with the accumulation of cellular exudate in alveoli
- Severe Phase – Pulmonary lesions reach a peak at around 14 days with dense bilateral infiltration and a large amount of cellular exudate
- Dissipative Phase – after 14 days, gradual absorption of lesions occurs (may occur earlier if disease course shorter)
- When followed over time, a pattern to stages of disease emerges (Li, M et al 2020)
- Role in Diagnosis:
- High sensitivity (97%), but low specificity (25%) to detect disease in a Wuhan epidemic context (Ai, T et al. 2020)
- A small study of CT-radiologists were able differentiate COVID from non-COVID viral pneumonias (Bai, HX et al. 2020), however they had a wide range of sensitivity (67-93%) and specificity (7-100%)
- Changes can be seen in asymptomatic patients (Shi, H et al. 2020) and be absent early in disease (Rodriguez JCL. et al 2020)
- High sensitivity (97%), but low specificity (25%) to detect disease in a Wuhan epidemic context (Ai, T et al. 2020)
- Ultrasonography
- Clinicians must consider infection control and prevention of transmission via contact with ultrasound machine when assessing COVID-19 patients
- Lung ultrasound
- Role is still being defined but no specific COVID-19 findings are yet noted from case reports (Soldati G et al. 2020)
- Cases exhibit presence of viral pneumonia with features including (Buonsenso, D et al. 2020):
- Irregular pleural line
- B-lines (may be irregular and even confluent)
- Patchy pattern with bilateral sparing
- Areas of white lung
- Subpleural consolidations
- Echocardiography, consider if:
- Hemodynamically unstable (e.g. cardiac causes, pericardial effusion, pulmonary embolism)
- Refractory hypoxaemia (e.g. diagnose patent foramen ovale using bubble study)
- Suspected right ventricular dysfunction (e.g. prolonged hypoxaemia/ pulmonary hypertension)
- Failed extubation (exclude cardiac cause)
- Neuroimaging
- Rare cases of COVID-19 encephalitis/ encephalopathy have been reported (Moriguchi, 2020; Poyiadji, 2020)
- CT brain
- symmetric hypoattenuation of medial thalami
- normal CT angiogram and CT venogram
- MRI brain
- May affect bilateral thalami, medial temporal lobes, hippocampi, and subinsular regions
- hyperintensity of affected regions on DWI and FLAIR
- hemorrhagic rim enhancing lesions
- Frontal hypoperfusion on perfusion imaging and strokes in patients with ARDS in ICU (Helms, 2020)
Other investigations
- Diagnostic bronchoscopy is not recommended due to risk of viral transmission from an AGP
- Nerve conduction studies if suspected Guillain-Barre Syndrome
MANAGEMENT – GENERAL
A set of ‘living guidelines’ for the management of patients with COVID-19 in Australia is published by the National COVID-19 Clinical Evidence Taskforce, and available from covid19evidence.net.au.
- Readers are encouraged to refer to the above resource for the latest treatment recommendations.
Resuscitation
- Ensure safety of the healthcare team in accordance with local hospital protocols
- All team members MUST don appropriate PPE equipment prior to attending to a patient, regardless of the urgency of the situation
- Droplet contact precautions are required for most patient care episodes, however, airborne/contact precautions are required for aerosol generating procedures (AGPs)
- Rapid, coordinated assessment and management with attention to immediate life threats, including:
- Severe hypoxaemia
- Treat with high flow oxygen to target SpO2 92-96%
- Avoid aerosol generating devices if possible, and ensure airborne/contact precautions if they are required
- Hypoxaemia an occur without significant respiratory distress
- Intubation is high risk if pre-oxygenation is inadequate
- Other life threats are uncommon (Yang et al, 2020), especially in the early stages of the disease, but may include:
- Severe hypoxaemia
- Post-intubation hypotension (e.g. patient may be dehydrated due to poor fluid intake and PEEP may markedly reduce venous return in combination with compliant lungs and sedative agents)
- Viral cardiomyopathy/ myocarditis (dysrhythmias, heart failure)
- Cytokine Storm Syndrome
- Pulmonary hypertension and right heart failure secondary to hypoxic pulmonary disease
- Multi-organ dysfunction (including acute kidney injury)
- Secondary bacterial infection
- Potential neurological involvement (e.g. affecting cardio-respiratory centers) (Li Y-C, 2020; Baig, 2020)
Specific therapies
- With the exception of remdesivir, for which there is some preliminary evidence of potential benefit (see novel & investigational therapies below), are no specific therapies with proven effectiveness currently available for the management of COVID-19
- Antimicrobial Therapy
- Empiric treatment of severe community acquired pneumonia and influenza according to local guidelines is usually appropriate as COVID-19 cannot be reliably distinguished from other viral and bacterial pneumonias on presentation
- Antimicrobial therapy should be assessed for de-escalation daily in light of clinical progress and microbiology results
- Oxygenation and ventilation strategies specific to COVID-19 are discussed below
Supportive care
- A comprehensive approach to supportive care is appropriate for all intensive care patients (e.g. FAST HUGS IN BED Please approach) and should be guided by local protocols.
- Fluid management
- Use a conservative fluid management strategy
- Avoid positive fluid balance/ hypervolaemia
- Excessive negative fluid balance (e.g. diuretics) could contribute to AKI and the need for CRRT (anecdotal experience from UK centers)
- Fluid resuscitation may be required in the early phase, especially prior to intubation and initiation of positive pressure ventilation (to avoid hypotension from impaired venous return)
- Analgesia/ sedation
- RASS 0 to -2 is ideal as over-sedation is harmful for intubated ICU patients
- Deeper sedation may be required to ensure safety, due to the risk of unplanned extubation and virus transmission
- Thromboprophylaxis
- Chemical prophylaxis (e.g. enoxaparin) according to ICU protocols
- COVID-19 patients may be at greater risk of venous-thromboembolism (VTE), DIC, and clotting of extracorporeal circuits (e.g. CRRT).
- A 20-50% rate of VTE in critically unwell patients has been observed in some ICU cohorts (see Complications, below)
- National COVID-19 clinical evidence taskforce guidelines (11 June 2020) recommend considering higher than usual doses of chemoprophylaxis in critically unwell patients (e.g. enoxaparin 40mg twice daily in patients with normal renal function or 40mg once daily in those with impaired renal function).
- Head up positioning (30-45°) to improve oxygenation and reduce risk of ventilator associated pneumonia
- Ulcer prophylaxis (follow local protocols for gastric acid suppression)
- Glucose control
- Skin and eye care (including pressure injury and review of line sites, especially if prone positioning used)
- Indwelling urinary catheters, nasogastric tubes
- Bowel care (e.g. laxatives)
- Environment (e.g. negative pressure room, optimise for delirium management)
- De-escalation (discuss goals of care early, de-escalate all of the above interventions (including removal of central lines) promptly once no-longer required)
- Psychosocial support to patient, staff, and family
- Vitally important to COVID-19 patients, as staff are potentially at risk of transmission, patient visitation is restricted and family may need to self-isolate or may also be ill.
- Provide families with frequent updates over phone/ video calls
- Enable communication between patient can family using diaries, communication boards, and technologies (e.g. telephone, video calls)
- Staff well being checks, use of PPE buddies,
Seek and treat complications
- Common complications
- Myocarditis/Cardiomyopathy up to 33% of critically ill cases (Arentz M. et al 2020; Ruan, Q. et al. 2020)
- Acute Kidney Injury: up to 8% of all cases (Rodriguez-Morales AJ. et al. 2020), likely higher in the critically ill
- May reflect excessive diuresis, microthrombi in kidneys, or other mechanisms
- Thrombosis
- In a Dutch study of 184 ICU patients with COVID pneumonia, 57% developed symptomatic venous thromboembolism despite prophylactic anticoagulation (Klok, F. et al, 2020; Klok F. et al, 2020 (2))
- Disseminated Intravascular Coagulation: reported in 71% of non-survivors in a case study (Tang N. et al. 2020)
- In a Dutch study of 184 ICU patients with COVID pneumonia, 57% developed symptomatic venous thromboembolism despite prophylactic anticoagulation (Klok, F. et al, 2020; Klok F. et al, 2020 (2))
- Secondary bacterial infection or co-infection
- Uncommon
- Acute Liver Injury (Xu L. et al 2020; Bangash MN. et al. 2020)
- Rhabdomyolysis (Jin M. et al. 2020)
Disposition and referrals
- Alfred Hospital Guidelines: Mandatory ICU review for all patients with:
- Oxygen >8L/min via face mask
- FiO2 >0.5 on HFNC
- All patients commenced on NIV
- All patients need early consideration of Goals of Care
- Specialist teams which may need to be involved:
- Infectious Diseases
- Respiratory
- Cardiology (if cardiac complications of COVID present)
- Other teams as required for patient comorbidities and complications
MANAGEMENT – OXYGENATION AND VENTILATION
Oxygenation strategies
- Usual oxygen therapy targets apply, e.g.
- SpO2 92-96% for most patients
- SpO2 88-92% for patients with COPD and CO2 retention
- Expert clinicians may choose other targets tailored to the individual patient (e.g. in some patients with refractory hypoxaemia, a lower target may be chosen to avoid the requirement for more invasive therapies and their associated risks)
- Oxygen devices that can be used with droplet/contact precautions in most settings (local protocols may differ):
- Nasal prongs (Standard)
- Face mask (e.g. Hudson)
- Non-rebreather mask (with reservoir bag)
- Venturi mask
- High-flow nasal cannula (HFNC) oxygen and Non-invasive ventilation (NIV)
- Current guideline recommendations
- Current Australian guidelines (v8.1) support the use of HFNC in acutely hypoxic patients, as does WHO guidance (WHO 2020/3/12)
- Current Australian guidelines (v8.1) recommend use of NIV with appropriate precautions against aerosol transmission. Note that earlier versions of the guideline recommended against the use of NIV.
- Risks of viral transmission
- Both HFNC and NIV have the potential to generate dispersal jets containing droplet or aerosolized viral particles (Hui DS. et al. 2019; Hui DS. et al 2009; ; Hui DS. et al. 2006))
- The use of NIV was associated with an increased risk of healthcare worker infection during the SARS epidemic (Raboud J. et al. 2010)
- HFNC may increase the distance travelled by droplet particles during coughing (Loh et al. 2020) however there is insufficient clinical evidence that HFNC increases the risk of viral transmission.
- Current Australian guidelines (v8.1) recommend that HFNC and NIV can be used in negative pressure rooms, single rooms or cohorted COVID areas and to avoid its use in shared wards, interhospital transport or Emergency Department cubicles.
- Potential benefit
- In patients with ARDS, the FLORALI trial found HFNC to be associated with a lower mortality than NIV (Frat et al. 2015), particularly in those with an FiO2:PaO2 ratio ≤ 200mmHg. NIV was also associated with increased mortality in the observational LUNG-SAFE trial (Bellani G. et al. 2020). In MERS cases there was a high failure rate from the use of BiPAP (Alraddadi BM. et al. 2019)
- It is uncertain what role NIV has in patients with SARS-CoV-2 infection presenting with other conditions in which NIV is typically indicated, such as exacerbation of chronic obstructive pulmonary disease (COPD) (Osadnik CR. et al. 2017) or cardiogenic pulmonary oedema (Berbenetz N. et al. 2019)
- A suggested approach
- Only use HFNP and NIV with appropriate airborne/contact precautions
- Patients who are COVID-19 positive but their comorbid conditions (e.g. OSA/COPD) are a major contributor to their respiratory illness may receive these therapies if they usually benefit from HFNP/NIV.
- NIV use in patients with COVID-19 should be used cautiously. CPAP may be the modality of choice for treatment of hypoxaemia and avoidance of excessive tidal volumes. Failure to improve (e.g. improved oxygenation, respiratory rate (e.g. <30/min), or work of breathing) over 1-2 hours suggests that ongoing NIV is unlikely to be beneficial.
- CPAP may be useful for optimisation of oxygenation prior to planned intubation in hypoxic CVOID19 patients
- HFNP and NIV use should not delayed intubation and initiation of mechanical ventilation if indicated
- HFNP and NIV patients require close monitoring as removal of the device or oxygen disconnection may result in life-threatening hypoxaemia
- Current guideline recommendations
Intubation
- Refer to the Safe Airway Society Consensus Guideline (Brewster et al, 2020) for principles and the Alfred ICU intubation guideline for an example of a context-specific local protocol.
- Indications
- Early intubation has been recommended based on early COVID-19 experience in China, that some patients progress to refractory hypoxaemia rapidly, and an awareness that preparation for intubation (an AGP) takes longer given the need for airborne/contact precautions
- However, reports from the UK/Europe/USA experience of the COVID-19 pandemic suggest that some patients who have severe hypoxaemia can avoid intubation if they are undistressed and otherwise stable (awake proning is being used in some centers)
- Pre-oxygenation
- Should involve a tight-fitting mask, e.g. BVM apparatus, CPAP mask attached closed circuit, Mapelson/ Waters apparatus.
- Patients refractory to pre-oxygenation (e.g. unable to achieve SpO2 >90-95%) likely have “shunt” physiology and may require CPAP/PPV for preoxygenation
- The intubation procedure itself is of high risk to clinical staff. Risk of viral transmission can be reduced by:
- Airborne/contact PPE precautions
- Avoid HFNC and nasal cannula under a mask due to risk of leak/ aerosolization
- Use of viral filter on BVM/ ventilators
- Rapid sequence induction with avoidance of BVM ventilation (unless required for re-oxygenation)
- Avoid cricoid pressure (may stimulate cough/ vomiting and worsen view)
- Routine use of videolaryngoscopy
- Avoiding auscultating the chest post-intubation
- There are wide-spread reports of post-intubation instability and deterioration in critically ill COVID-19 patients, possibly related to:
- Refractory hypoxaemia with in-effective pre-oxygenation
- Effect of PPV/ PEEP on venous return and/or right heart function
- Reduce risk of aerosol generation post-intubation:
- ETT cuff pressure checks to prevent cuff leak
- Use in-line suctioning
- Tight circuit connections (some centers tape connections)
Mechanical Ventilation
- Most guidance is based on extrapolation from ARDS management and previous experience with severe coronavirus disease (e.g. SARS, MERS, and COVID-19)
- A protective lung ventilation strategy is recommended
- Tidal volumes (VT) of 4-6 mL/kg PBW
- Aim for plateau pressures (Pplat) < 30 cmH20
- Allow permissive hypercapnia unless significant acidemia (e.g. pH <7.15) or otherwise contra-indicated
- The role of positive-end expiratory pressure (PEEP) is controversial
- The original ANZICS guidelines (version 1) recommended higher PEEP levels (e.g. >15 cmH20) for ongoing hypoxia. However, much lower PEEP settings may be appropriate for the subset of COVID19 patients (e.g. “L phenotype”) with highly compliant lungs as little evidence of “recruitability”.
- Australian guidelines (v2) now make no PEEP recommendations.
- In general, PEEP should be incrementally adjusted using the minimum PEEP required for optimisation. How to best optimise PEEP is controversial, though the simplest approach is to adjust to target SpO2. The ARDSNet ventilation strategy provides a step-wise approach to adjusting PEEP settings based on the FiO2 required. An example of a guide to titrated FiO2/PEEP settings is available here.
Other strategies for refractory hypoxaemia
- Ensure simple measures considered:
- Patent well positioned ETT of an appropriate size with appropriate ventilator settings and oxygen flow
- Chest physiotherapy and patient positioning
- Optimise fluid balance (e.g. diuretics, renal replacement therapy)
- Prone positioning
- Widely used globally with reported benefits in oxygenation
- For mechanically ventilated adults with COVID-19 and hypoxaemia despite optimising ventilation, consider prone positioning (Australian guidelines (v2.1)).
- Neuromuscular blockade
- May be required to optimise mechanical ventilation and decrease oxygen consumption
- Intermittent dosing should be used as first line in preference to continuous infusions
- Inhaled Nitric Oxide (iNO) and pulmonary vasodilators (e.g. prostacyclin)
- Not recommended for routine use
- A trial of iNO is reasonable for refractory hypoxaemia, as abnormal hypoxic vasoconstriction play place a role
- Need to consider risk of aerosol generation and circuit disconnections and their implications for viral transmission if used
- Recruitment manoeuvres
- May be beneficial for patients with evidence of “recruitable lung”
- For mechanically ventilated adults with COVID-19 and hypoxaemia despite optimising ventilation, consider using recruitment manoeuvres (Australian guidelines (v2.1))
- If recruitment manoeuvres are used, do not use staircase or stepwise (incremental PEEP) recruitment manoeuvres (Australian guidelines (v2.1)).
- Bronchoscopy
- Therapeutic bronchoscopy may relieve sputum plugging
- Bronchoscopy is considered an AGP, patients should have adequate neuromuscular blockade and airborne/contact precautions are required
- Extracorporeal membrane oxygenation (ECMO)
- The utility of ECMO for COVID19 is uncertain and there are concerns about the resource implications of ECMO in the context of a global pandemic
- Currently, WHO recommends its use be “considered” in centres with appropriate expertise (WHO, 13/3/2020).
- Australian guidelines (v2.1) recommend that mechanically ventilated adults with COVID-19 and refractory hypoxaemia (despite optimising ventilation, use of rescue therapies and proning), should be considered for venovenous extracorporeal membrane oxygenation (VV ECMO) (e.g. referral to an ECMO center).
Tracheostomy
- Tracheostomy is an AGP and it’s role in the context of a COVID19 pandemic is uncertain.
Extubation
- Extubation is an AGP, primarily due to the risk of patient coughing
- Timing
- Optimize timing to decrease the likelihood of needing High Flow Nasal Prongs (HFNP), Non Invasive Ventilation (NIV) or re-intubation following extubation
- Timing is further complicated by:
- Anecdotal reports of increased rates of extubation from airway oedema and even cardiovascular collapse (possible cardiomyopathy)
- Potential for late deterioration in COVID19 lung disease (e.g. transition from high compliance to low compliance lungs)
- Cuff leak tests have been recommended by some centers to guide decision making, however practitioners need to be aware that the cuff leak test is a potential AGPs.
- Procedure
- All team members should have PPE appropriate for airborne/ contact precautions
Refer to the Alfred ICU extubation guideline for an example of a local protocol for extubation
NOVEL AND INVESTIGATIONAL THERAPIES
The following therapies are under investigation for use in patients with SARS-CoV-2 infection. They lack the robust clinical evidence required to recommend for routine practice. They should only be used as part of a clinical trial with appropriate ethical approval until such time that further evidence of their safety and efficacy becomes available.
Clinicians should also refer to the National COVID-19 Clinical Evidence Taskforce ‘living guidelines’ for up to date recommendations.
Many large trials including are ongoing, including:
- World Health Organisation SOLIDARITY trial: multi-arm remdesivir, lopinavir/ritonavir, hydroxychloroquine, Interferon Beta-1A.
- REMAP-CAP: a multifactorial adaptive platform trial of community acquired pneumonia, which includes a pandemic response arm. Investigations include lopinavir/ritonavir, hydroxychloroquine, corticosteroids and immune therapies (Angus D. et al. 2020).
- RECOVERY: UK-based multifactorial adaptive platform trial, similar to REMAP-CAP (NCT04381936).
Antiviral Therapy
Possible benefit:
- Remdesivir
- Adenosine analogue and RNA-dependent RNA-polymerase inhibitor, required for viral replication (Lung J. et al. 2020). Not licensed by TGA and limited availability in Australia.
- Some evidence of effective use in MERS (de Wit E. et al. 2020).
- Not associated with reduced time to clinical improvement, mortality or viral clearance in a double blind, multicentre RCT (China, n=237) of hospitalised patients (Wang Y. et al. 2020). This trial was terminated early due to a lack of recruitment and may have been underpowered (further analysis on INTENSIVE).
- ACTT-1 trial (Beigel J et al. 2020): In preliminary results from this US-led RCT (n=1063), remdesivir for up to 10 days was associated with a shorter time to ‘clinical recovery’ of 11 (95% CI 9 to 12) vs.15 (95% CI 13-19) days. In patients requiring oxygen therapy (but not high-flow oxygen or mechanical respiratory support) 15-day mortality was reduced from 10.9 to 2.4% (HR 0.22, 95% CI 0.08 to 0.58). Final results including 28 day outcomes are awaited (further analysis on INTENSIVE).
- Further large RCTs are awaited as above.
Uncertain effect:
- The following currently have no no role in the clinical management of COVID-19 except in the context of well-designed clinical trials.
- Agents currently under investigation include:
- Lopinavir/Ritonavir (Kaletra)
- Antiretroviral agent developed for use in HIV infection.
- In an open label study of 199 patients in China, lopinavir/ritonavir was not associated with a reduction in mortality or time to clinical resolution of symptoms (Cao et al. 2020).
- Ribavirin
- Guanosine and adenosine analogue, used in other RNA viruses including hepatitis C virus.
- In a small, open label multicentre RCT (Hong Kong) use in combination with interferon beta-1b and lopinavir/ritonavir was associated with a shorter time to a SARS-CoV-2 PCR negative result. Due to the complex treatment regime the relative contribution of ribavirin is uncertain (Hung IF et al. 2020).
- Lopinavir/Ritonavir (Kaletra)
Low likelihood of benefit or potential for harm
- Chloroquine – In vitro data suggests that chloroquine can inhibit COVID-19 (Wang M. et al. 2020) however clinical trials have been associated with potential harm:
- ChloroCovid-19: RCT (Brazil; n=81) low vs. high dose chloroquine in hospitalised patients with suspected COVID-19, terminated early due to increased 14 day mortality with high dose chloroquine (39 vs. 15% OR 3.6; 95% CI 1.2-10.6; Borba et al. 2020)
- Hydroxychloroquine
- In a widely publicised, non-randomised, open label trial in combination with azithromycin, hydroxychloroquine was associated with reduced viral load (Gautret P. et al. 2020). Significant methodological concerns have been raised with this trial (Intson K. et al. 2020) and others have not been able to replicate this result (Molina et al. 2020). Further analysis of this trial on INTENSIVE.
- No benefit in mild to moderate disease based on an open label MRCT (China, n=150; Tang W. et al. 2020).
- Not associated with benefit two large US observational studies (Geleris J. et al. 2020; Rosenberg E. et al. 2020)
- May increase QTc especially in combination with azithromycin (Mercuro N et al. 2020).
- On June 5 the RECOVERY trial investigators announced a finding of no benefit although full results are awaited.
- Ongoing investigation in multiple trials including REMAP-CAP, SOLIDARITY (WHO) & RECOVERY (UK).
- SOLIDARITY continues to recruit
- REMAP-CAP has ceased recruitment in the UK.
- On June 15, the FDA (US) revoked emergency use authorization for hydroxychloroquine and chloroquine in COVID-19 and use in the US is no longer permitted outside of a clinical trial.
Corticosteroids
- Not recommended for routine use by national Guidelines
- Evidence lacking in SARS-CoV-2 infection
- Systematic review and meta-analysis of SARS & MERS coronavirus suggests increased mortality (RR 2.1, 95% CI 1.1-3.9; Yang Z. et al. 2020)
- Previous studies using steroids in SARS found that they may increase viral shedding (Nelson L. et al 2004)
- Proponents argue that there is a role for corticosteroids (Villar et al, 2020) in:
- COVID-19 ARDS to prevent pulmonary fibrosis
- COVID-19 cytokine storm
- Corticosteroids is one of the therapeutic arms of the REMAP-CAP, RECOVERY and SOLIDARITY trials and may be used in the treatment of septic shock as a vasopressor- sparing agent.
- On 16 June RECOVERY announced preliminary results suggesting mortality benefit, but full results including peer review are awaited.
Immunotherapy
- Severe COVID19 disease may have features of cytokine storm syndrome/ hemophagocytic lymphohistiocytosis (HLH). Immune modulator therapy has been used in HLH and has been hypothesised to modulate severe COVID-19 (Mehta et al. 2020)
- Examples include:
- Interferon-beta (see trial of combination with ribavirin above, Hung IF et al. 2020)
- Anti-interferon-gamma (emapalumab) – trials ongoing (NCT04324021)
- Anakinra (IL-1) – trials ongoing (NCT04324021)
- Anti-IL6 Agents (Toscilizumab, Siltuximab)
- Monoclonal antibody to IL-6.
- Case reports of use (Colaneri M. et al. 2020, Capra R. et al. 2020) with trials ongoing; (NCT04322773; NCT04306705; NCT04322773)
- Convalescent plasma:
- Removal of plasma of patients recently recovered from COVID-19 and transfusing it into current patients to elicit immune response. Also hyperimmune fractionated immunoglobulin.
- In a small RCT (n=103) use was associated with clinical improvement in patients who were not invasively ventilated, as well as a shorter time to viral clearance (Li L. et al. 2020).
- “Living” Cochrane review (Valk S. et al. 2020).
- Further trials ongoing.
Other
Empiric Anticoagulation and Thrombolysis
- COVID-19 may be complicated by a pro-thrombotic coagulopathy and its presence is associated with a high mortality (Tang, N et al 2020, Yin, S. et al 2020)
- Use of prophylactic heparin/low molecular weight heparin is considered in the regular care of Intensive Care patients (see ‘Supportive Care’ above) and is considered standard of care for critically unwell patients in the absence of contraindications.
- Therapeutic anticoagulation
- Due to the high incidence of thromboembolic events, some groups suggest the use of therapeutic doses of heparin/low molecular weight heparin (Lin, L et al. 2020, Tang, N et al 2020), however prospective evidence is lacking and caution is essential given the risk of bleeding complications.
- Heparin may have many putative effects in COVID-19 (Thachil, J. 2020, Tang, N et al 2020) including reduced microvascular and large vessel thrombosis, anti-inflammatory effects and endothelial protection.
- Other experimental therapies (use should be limited to clinical trials):
- tPA infusion (Wang J. et. al. 2020) and inhaled plasminogen (Wu Y et al. 2020) and complement inhibitors (eculizumab, as used for thrombotic microangiopathies; Campbell C & Kahwash R. 2020) have been reported.
Renin-Angiotensin-Aldosterone System (RAAS) Inhibitors and Modulators (Vaduganathan et al, 2020)
- ACE2 physiologically counters RAAS activation and functions as a receptor for SARS-CoV-1 and SARS-CoV-2 viruses.
- Preclinical studies have suggested that RAAS inhibitors may increase ACE2 expression, but it is unknown if this occurs in humans or is clinically relevant.
- Clinical trials are under way to test the safety and efficacy of RAAS modulators, including recombinant human ACE2 and the ARB losartan in Covid-19
- Discontinuation of RAAS Inhibitors (e.g. AT2 antagonists, ACE inhibitors) in patients with a pre-existing indication is not recommended in patients with suspected or confirmed COVID-19 (see also Prognosis below)
High Altitude Pulmonary Edema (HAPE) therapies
- A role for HAPE therapies in treatment of COVID-19 has been proposed, based on parallels between the two diseases, including: Nifedipine, Acetazolamide, and Sildenafil (Solaimanzadeh, 2020)
- However, the pathophysiology of the two conditions is very different (hypoxic pulmonary vasoconstriction vs. viral induced parenchymal injury)
The risk/benefit of these agents for the treatment of COVID-19 is unknown and there is the potential that treatments used for HAPE could be harmful in COVID-19, despite clinical and radiological similarities (Luks A. et al. 2020).
PROGNOSIS
Length of Stay
- Median hospital length of stay in China:
- 12 days (IQR 10-14) when applied across all severity groups
- 14.5 days (IQR 11-19) in those admitted to ICU, requiring mechanical ventilation or who died (Guan W. et al. 2020)
Mortality
- Chinese CDC data suggests a case-fatality rate of of 0.25-3% (Wilson N. et al. 2020)
- Estimates vary by location of disease – range from ~1.5% in South Korea (KCDC 2020), 2.3% in China (Wu Z. & McGoogan J. 2020, to 7.2% in Italy (Onder G. et al. 2020).
- Case-fatality rates may be higher in disease epicentres due to system breakdown leading to a higher mortality and/or reduced resources to undertake screening programs of ambulatory patients (Mizumoto K. & Chowell G. 2020)
- Fatality estimates may also be influenced by:
- Population demographics (Italy has an older age distribution to China),
- Differences in screening programs, as above, may underestimate the denominator and overestimate fatality rates if mild cases are not tested
- Different definitions of COVID-19 related deaths (eg. Italy defined COVID-19 deaths as all deaths occurring in SARS-CoV-2 positive patients independent of other pre-existing diseases which may have contributed to death; Onder G. et al. 2020)).
- COVID-19 appears to have a lower fatality rate than SARS (9.6%) and MERS (34.4%) however it has led to numerically more deaths due to its greater spread. (Wu Z. & McGoogan J. 2020)
- In-hospital cardiac arrest outcomes are very poor in COVID-19 patients with a respiratory cause (Shao et al, 2020)
- Of 136 COVID-19 patients in Wuhan, China, only one had favourable neurological recovery at 30 days after IHCA, despite 89% of patients receiving CPR <1min. Most had asystole as an initial rhythm (90%) and a respiratory cause (87.5%).
Disease Severity
Risk factors for severity of disease and fatality:
- Age (Wu Z. & McGoogan J. 2020)
- Any age can develop a severe respiratory failure
- Fatality is correlated to age with highest mortality 14-20% in ≥80 years
- Fewer children tend to suffer severe disease (5.2%) or critical disease (0.2%) (Ludvigsson, J. 2020)
- Until March 2020 there were no reported deaths directly from COVID in children <9 years old, however there are now emerging reports of infant deaths in news media (Sibthorpe, C. 2020)
- Odds of 1.10 increase in death per year in hospitalised patients (Zhou F et al. 2020)
- Gender
- Females appear to be less severely affected than males. This effect may increase with age (Singh et al. 2020 [pre-print]).
- Disease severity
- High SOFA score (Zhou F et al. 2020).
- qSOFA is not discriminatory due to a high incidence of tachypnea even with mild disease, and the preservation of mentation and blood pressure even in severe disease (Ferreira M. et al. 2020).
- Case fatality rate is approximately 50% in critical cases (Wu Z. & McGoogan J. 2020)
- High SOFA score (Zhou F et al. 2020).
- Comorbidity
- Presence of co-existing disease is more common in patients with severe disease (Guan W. et al. 2020), including:
- Cardiovascular disease
- Diabetes
- Chronic Respiratory disease
- Hypertension
- Cancer
- In Italy, of those who died (Onder G. et al. 2020):
- 0.8% had no disease
- 25.1% had a single disease
- 25.6% had two diseases
- 48.5% had 3 or more diseases
- Hypertension and cardiovascular disease are associated with severe disease and mortality, however use of ACEI/ARB does not appear to independently increase risk (Jarcho J. et al. 2020)
- Presence of co-existing disease is more common in patients with severe disease (Guan W. et al. 2020), including:
- Laboratory features associated with severity/mortality (Zhou F. et al. 2020):
- D-Dimer > 1ug/mL on admission (OR 18)
- Others include:
- Leukocytosis with lymphopenia
- Anaemia
- Thrombocytopenia
- Elevated ALT, LDH
- Ferritin and IL-6
- Severe disease may resemble “cytokine storm syndrome” or “hemophagocytic lymphohistiocytosis”; HLH with hyperferritinemia. (Mehta P. et al. 2020) (Cron, R., Chatham, W 2020)
- hs-Troponin or CK-MB (Shi S. et al. 2020)
- Elevated CRP or procalcitonin
Special populations
- Children (Ludvigsson J. 2020)
- Appear to develop milder disease with a very low risk of mortality from COVID-19
- As of April, 2020 there have only been reported a handful of paediatric deaths worldwide in association with SARS-CoV-2 infection
- Pregnancy and neonates
- Vertical transmission rates appear low (as with SARS-CoV) (Zhu H. et al. 2020; Chen H. et al. 2020)
- SARS (SARS-CoV) was associated with high incidence of neonatal adverse events, however limited case series (so far) suggests the rate of adverse events may be low in SARS-CoV-2 (Qiao J. 2020; Rasmussen S. et al. 2020)
- SARS-CoV-2 infection does not appear to be associated with an increased rate of maternal adverse outcome, unlike the H1N1 influenza pandemic in which pregnant patients were over-represented in mortality (Rasmussen S. et al. 2020)
- Immunocompromised (Weinkove R. et. al. 2020)
- Little direct evidence on the impact of COVID-19 infection in immunocompromised patients.
- May be at increased risk of severe COVID-19 based on experience with other respiratory viruses.
- Differential diagnosis may be difficult given frequency of pulmonary infiltrates and potential for co-infection.
- Impact of treatment interruption from population transmission control also uncertain.
Immunity
- Humoural immunity occurs with presence of IgM and IgG antibodies in plasma following exposure.
- In one study of 173 patients, median time to seroconversion was 11-12 days.
- 94% of patients were IgM positive and 80% of patients were IgG positive two weeks after illness onset. (Zhao J. et al. 2020).
- Duration of humoural response unknown, however post SARS-CoV infection 100% of patients had positive IgG titres up to 16 months, with approximately 90% still IgG positive at two years, declining to 50% at four years. (Lin Q. et al. 2020)
PREVENTION
Measures used to prevent SARS-CoV-2 transmission in healthcare settings include:
- Individual level approaches (Ferioli et al, 2020)
- droplet/ contact precautions for all staff involved in COVID19 patient interactions
- airborne/ contact precautions for all staff involved in AGPs, which should be performed in a closed room (ideally with negative pressure)
- Hand hygiene
- Avoid touching one’s face
- minimise equipment use (e.g. avoid stethoscope use)
- System level approaches
- Clear, consistent communication and education of staff and patients
- Regular cleaning of environmental surfaces
- Equipment cleaning
- appropriate room ventilation (e.g. increased ventilation rate, using natural ventilation, avoiding air recirculation, and negative pressure rooms for AGPs)
- Social distancing of staff (e.g. at lunch breaks)
- Cohorting of patients and patient care areas (including separate locations for triage and care)
- High risk staff members (e.g. age >65 years, pregnant, immunocompromised) not assigned to care of COVID-19 patients
- Appropriate rostering and shift breaks
- limiting the number of healthcare workers involved in care
- Telemedicine and use of remote technologies
- Prioritise PPE availability and SARS-CoV-2 testing for healthcare, transportation, safety, security, and infrastructure workers
- Vaccine development (Lurie, 2020)
CONTROVERSIES
There are many controversies concerning our understanding of COVID-19, including issues relevant to assessment and management:
- Potential for airborne transmission
- The existence of L and H phenotypes and their implications for ICU management (see Is COVID-19 ARDS? What about lung compliance?)
- Potential for neurotropic effects of the virus (e.g. effects on cardio-respiratory centers and role in cardiac arrest and response to hypoxaemia)
- Role of HFNC and NIV
- Thresholds and timing of interventions, including different oxygenation strategies and intubation (see “Silent hypoxaemia” and COVID-19 intubation)
- How to maintain oxygenation during intubation
- Appropriate PEEP settings in mechanical ventilation (see COVID-19: “To PEEP, or not to PEEP”?)
- Utility of specific therapies (e.g. antivirals, steroids, immunotherapy) (see Novel drug therapies and COVID-19 clinical trials)
- Extubation and tracheostomy timing and procedures
- Therapies for refractory hypoxaemia, including vasodilators (iNO), recruitment manoeuvres, and ECMO
- Care of isolated patients and families, including including compassionate care at the end-of-life (Wakam, 2020)
- Long term outcomes and recovery of COVID-19 survivors
- Pandemic resourcing and ethics
- Staff wellbeing and moral distress, and the mental health burden of the COVID-19 pandemic
- Rapid implementation of device and process innovations (see MacGyverism and “hacking COVID-19”)
Harms caused by the socioeconomic effects and disruption of usual services from pandemic precautions (e.g. disruption of elective health services, lack of employment, impacts on education, domestic violence) (Rosenbaum, 2020).
REFERENCES
LITFL
- Acute Respiratory Distress Syndrome (ARDS)
- Acute Respiratory Distress Syndrome Definitions
- COVID-19: Keeping the baby in the bath (7-part series on COVID-19 critical care controversies)
- Driving pressure
- Imaging of COVID-19 pneumonia: a critical care perspective
- Improving oxygenation in ARDS
- LITFL Coronavirus resources
- Lung Ultrasound in COVID-19
- Neuromuscular blockade in ARDS
- Positive End- Expiratory Pressure (PEEP)
- Post-intubation hypoxia
- Prone Position and Mechanical Ventilation
- Protective Lung Ventilation
- Open Lung Approach To Ventilation
Journal articles and guidelines
- Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases [published online ahead of print, 2020 Feb 26]. Radiology. 2020;200642. doi:10.1148/radiol.2020200642 [Pubmed]
- Alraddadi BM, Qushmaq I, Al-Hameed FM, et al. Noninvasive ventilation in critically ill patients with the Middle East respiratory syndrome. Influenza Other Respir Viruses. 2019;13(4):382–390 [Pubmed]
- Angus DC, Berry S, Lewis RJ, et al. The Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia (REMAP-CAP) Study: Rationale and Design [published online ahead of print, 2020 Apr 8]. Ann Am Thorac Soc. 2020;10.1513/AnnalsATS.202003-192SD. doi:10.1513/AnnalsATS.202003-192SD [pubmed]
- ANZICS COVID Working Group (2020). The Australian and New Zealand Intensive Care Society (ANZICS) COVID-19 Guidelines. Version 2. 15/4/2020. https://www.anzics.com.au/wp-content/uploads/2020/03/ANZICS-COVID-19-Guidelines-Version-1.pdf Last accessed 17/4/2020 [No Pubmed Available]
- Arentz M, Yim E, Klaff L, et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State [published online ahead of print, 2020 Mar 19]. JAMA. 2020;e204326. doi:10.1001/jama.2020.4326 [pubmed]
- Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility [published online ahead of print, 2020 Apr 24]. N Engl J Med. 2020;NEJMoa2008457. doi:10.1056/NEJMoa2008457 [pubmed]
- Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT [published online ahead of print, 2020 Mar 10]. Radiology. 2020;200823. doi:10.1148/radiol.2020200823 [pubmed]
- Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020 Mar 13 [Online ahead of print] [pubmed]
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 – Preliminary Report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764. doi:10.1056/NEJMoa2007764 [pubmed]
- Borba MGS, Val FFA, Sampaio VS, et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(4):e208857. Published 2020 Apr 24. doi:10.1001/jamanetworkopen.2020.8857 [pubmed]
- Bouadma L, Lescure FX, Lucet JC, Yazdanpanah Y, Timsit JF. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46(4):579–582. doi:10.1007/s00134-020-05967-x [pubmed]
- Bourouiba L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA. Published online March 26, 2020. doi:10.1001/jama.2020.4756 [pubmed]
- Bourouiba L. IMAGES IN CLINICAL MEDICINE. A Sneeze. N Engl J Med. 2016;375(8):e15. doi:10.1056/NEJMicm1501197 [pubmed]
- Buonsenso D, Piano A, Raffaelli F, Bonadia N, de Gaetano Donati K, Franceschi F. Point-of-Care Lung Ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24(5):2776–2780. doi:10.26355/eurrev_202003_20549 [pubmed]
- Brewster DJ, Chrimes N, Do TB, et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group [published online ahead of print, 2020 May 1]. Med J Aust. 2020;10.5694/mja2.50598. doi:10.5694/mja2.50598 [pubmed]
- Campbell CM, Kahwash R. Will Complement Inhibition be the New Target in Treating COVID-19 Related Systemic Thrombosis? [published online ahead of print, 2020 Apr 9]. Circulation. 2020;10.1161/CIRCULATIONAHA.120.047419. doi:10.1161/CIRCULATIONAHA.120.047419 [pubmed]
- Capra R, De Rossi N, Mattioli F, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia [published online ahead of print, 2020 May 13]. Eur J Intern Med. 2020;10.1016/j.ejim.2020.05.009. doi:10.1016/j.ejim.2020.05.009 [pubmed]
- Chan JF, Yip CC, To KK, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens [published online ahead of print, 2020 Mar 4]. J Clin Microbiol. 2020;JCM.00310-20. doi:10.1128/JCM.00310-20 [pubmed]
- CDC. Interim Guidelines for Collecting, handling and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19). Last update 24/3/2020) https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html (Last accessed 30/3/2020 [No Pubmed Available]
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038]. Lancet. 2020;395(10226):809–815. doi:10.1016/S0140-6736(20)30360-3 [pubmed]
- Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi:10.1002/jmv.25681 [pubmed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7 [pubmed]
- Colaneri M, Bogliolo L, Valsecchi P, et al. Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms. 2020;8(5):E695. Published 2020 May 9. doi:10.3390/microorganisms8050695 [pubmed]
- Communicable Diseases Network Australia (2020). Coronavirus Disease 2019 (COVID-19) CDNA national guidelines for public health units v. 2.7 [24th April 2020]. Department of Health, https://www1.health.gov.au/internet/main/publishing.nsf/Content/cdna-song-novel-coronavirus.htm (last accessed 27/4/2020) [No Pubmed Available]
Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi:10.2807/1560-7917.ES.2020.25.3.2000045 [pubmed] - Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi:10.1038/s41564-020-0695-z [pubmed]
- Cron RQ, Chatham WW. The Rheumatologist’s Role in Covid-19 [published online ahead of print, 2020 Mar 24]. J Rheumatol. 2020;jrheum.200334. doi:10.3899/jrheum.200334 [pubmed]
- de Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020;117(12):6771–677 [pubmed]
- Döhla M, Boesecke C, Schulte B, et al. Rapid point-of-care testing for SARS-CoV-2 in a community screening setting shows low sensitivity [published online ahead of print, 2020 Apr 18]. Public Health. 2020;182:170–172. doi:10.1016/j.puhe.2020.04.009 [pubmed]
- ELSO: ECMO in COVID-19. 24/3/2020. https://www.elso.org/covid19. Last accessed 29/3/2020 [No pubmed available]
- Farkas, J (2020). The Internet Book of Critical Care: COVID-19. 2/3/2020. https://emcrit.org/ibcc/COVID19/ Last updated 26/3/2020, Last accessed 27/3/2020 [No Pubmed available]
- Ferioli M, Cisternino C, Leo V, Pisani L, Palange P, Nava S. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev. 2020;29(155):200068. Published 2020 Apr 3. doi:10.1183/16000617.0068-2020 [pubmed]
- Ferreira M, Blin T, Collercandy N, et al. Critically ill SARS-CoV-2-infected patients are not stratified as sepsis by the qSOFA. Ann Intensive Care. 2020;10(1):43. Published 2020 Apr 19. doi:10.1186/s13613-020-00664-w [pubmed]
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. [pubmed]
- Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? [published online ahead of print, 2020 Apr 14]. Intensive Care Med. 2020;1‐4. doi:10.1007/s00134-020-06033-2 [pubmed]
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [published online ahead of print, 2020 Mar 20]. Int J Antimicrob Agents. 2020;105949. doi:10.1016/j.ijantimicag.2020.105949 [pubmed]
- Gandhi M, Yokoe DS, Havlir DV. Asymptomatic Transmission, the Achilles’ Heel of Current Strategies to Control Covid-19 [published online ahead of print, 2020 Apr 24]. N Engl J Med. 2020;10.1056/NEJMe2009758. doi:10.1056/NEJMe2009758 [pubmed]
- Geleris J, Sun Y, Platt J, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19 [published online ahead of print, 2020 May 7]. N Engl J Med. 2020;NEJMoa2012410. doi:10.1056/NEJMoa2012410 [pubmed]
- Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study [published online ahead of print, 2020 Mar 26]. Clin Infect Dis. 2020;ciaa330. doi:10.1093/cid/ciaa330 [pubmed]
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China [published online ahead of print, 2020 Feb 28]. N Engl J Med. 2020;10.1056/NEJMoa2002032. doi:10.1056/NEJMoa2002032 [pubmed]
- Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020 [published online ahead of print, 2020 Apr 10]. Emerg Infect Dis. 2020;26(7):10.3201/eid2607.200885. doi:10.3201/eid2607.200885 [pubmed]
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi:10.1002/path.1570 [pubmed]
- Helms J, Kremer S, Merdji H, et al. Neurologic Features in Severe SARS-CoV-2 Infection [published online ahead of print, 2020 Apr 15]. N Engl J Med. 2020;NEJMc2008597. doi:10.1056/NEJMc2008597 [pubmed]
- Henry BM. COVID-19, ECMO, and lymphopenia: a word of caution [published online ahead of print, 2020 Mar 13]. Lancet Respir Med. 2020;S2213-2600(20)30119-3. doi:10.1016/S2213-2600(20)30119-3 [pubmed]
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271‐280.e8. doi:10.1016/j.cell.2020.02.052 [pubmed]
- Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China [published online ahead of print, 2020 Mar 4]. Sci China Life Sci. 2020;10.1007/s11427-020-1661-4. doi:10.1007/s11427-020-1661-4 [pubmed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30;:]. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5 [pubmed]
- Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial [published online ahead of print, 2020 May 8]. Lancet. 2020;S0140-6736(20)31042-4. doi:10.1016/S0140-6736(20)31042-4 [pubmed]
- Iacobucci G. Sixty seconds on . . . anosmia. BMJ. 2020;368:m1202. Published 2020 Mar 24. doi:10.1136/bmj.m1202 [pubmed]
- Iba T, Levy JH, Warkentin TE, et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17(11):1989–1994. doi:10.1111/jth.14578 [pubmed]
- Intson K, Kumar S, Botta A, Neckles R, Leung C, Jawaid A. An independent appraisal and re-analysis of hydroxychloroquine treatment trial for COVID-19. Swiss Med Wkly. 2020;150:w20262. Published 2020 Apr 29. doi:10.4414/smw.2020.20262 [pubmed]
- Jarcho JA, Ingelfinger JR, Hamel MB, D’Agostino RB Sr, Harrington DP. Inhibitors of the Renin-Angiotensin-Aldosterone System and Covid-19 [published online ahead of print, 2020 May 1]. N Engl J Med. 2020;10.1056/NEJMe2012924. doi:10.1056/NEJMe2012924 [pubmed]
- Kanne JP, Little BP, Chung JH, Elicker BM, Ketai LH. Essentials for Radiologists on COVID-19: An Update-Radiology Scientific Expert Panel [published online ahead of print, 2020 Feb 27]. Radiology. 2020 [pubmed]
- KCDC (2020) The updates on COVID-19 in Korea: https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030Last accessed 26/3/2020 [No pubmed available]
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19 [published online ahead of print, 2020 Apr 10]. Thromb Res. 2020;S0049-3848(20)30120-1. doi:10.1016/j.thromres.2020.04.013 [pubmed]
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis [published online ahead of print, 2020 Apr 30]. Thromb Res. 2020;S0049-3848(20)30157-2. doi:10.1016/j.thromres.2020.04.041 [pubmed]
- Kutter JS, Spronken MI, Fraaij PL, Fouchier RA, Herfst S. Transmission routes of respiratory viruses among humans. Curr Opin Virol. 2018;28:142–51. [pubmed]
- Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi:10.1016/j.ijantimicag.2020.105924 [pubmed]
- Lauer SA, Grantz KH, Bi Q, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application [published online ahead of print, 2020 Mar 10]. Ann Intern Med. 2020;M20-0504. doi:10.7326/M20-0504 [pubmed]
- Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304–309. [pubmed]
- Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks [published online ahead of print, 2020 Apr 3]. Nat Med. 2020;10.1038/s41591-020-0843-2. doi:10.1038/s41591-020-0843-2 [pubmed]
- Li L, Zhang W, Hu Y, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial [published online ahead of print, 2020 Jun 3]. JAMA. 2020;10.1001/jama.2020.10044. doi:10.1001/jama.2020.10044 [pubmed]
- Li M, Lei P, Zeng B, et al. Coronavirus Disease (COVID-19): Spectrum of CT Findings and Temporal Progression of the Disease [published online ahead of print, 2020 Mar 20]. Acad Radiol. 2020;S1076-6332(20)30144-6. doi:10.1016/j.acra.2020.03.003 [pubmed]
- Li Y-C, Bai W-Z, Hashikawa T. The neuroinvasiveness potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 Feb 27 [Epub ahead of print] [pubmed]
- Li Z, Yi Y, Luo X, et al. Development and Clinical Application of A Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis [published online ahead of print, 2020 Feb 27]. J Med Virol. 2020;10.1002/jmv.25727. doi:10.1002/jmv.25727 [pubmed]
- Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. doi:10.1080/22221751.2020.1746199 [pubmed]
- Lin Q, Zhu L, Ni Z, Meng H, You L. Duration of serum neutralizing antibodies for SARS-CoV-2: Lessons from SARS-CoV infection [published online ahead of print, 2020 Mar 25]. J Microbiol Immunol Infect. 2020;S1684-1182(20)30075-X. doi:10.1016/j.jmii.2020.03.015 [pubmed]
- Liu Q. & Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. [pubmed]
- Lu J, Gu J, Li K, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020 Jul. [pubmed]
- Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults [published online ahead of print, 2020 Mar 23]. Acta Paediatr. 2020;10.1111/apa.15270. doi:10.1111/apa.15270 [pubmed]
- Luks A, Freer L, Grissom C, et al. COVID-19 Lung Injury is Not High Altitude Pulmonary Edema [published online ahead of print, 2020 Apr 13]. High Alt Med Biol. 2020;10.1089/ham.2020.0055. doi:10.1089/ham.2020.0055 [pubmed]
- Lung J, Lin YS, Yang YH, et al. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase [published online ahead of print, 2020 Mar 13]. J Med Virol. 2020;10.1002/jmv.25761. doi:10.1002/jmv.25761 [pubmed]
- Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 Vaccines at Pandemic Speed [published online ahead of print, 2020 Mar 30]. N Engl J Med. 2020;10.1056/NEJMp2005630. doi:10.1056/NEJMp2005630 [pubmed]
- MacLaren G, Fisher D, Brodie D. Preparing for the Most Critically Ill Patients With COVID-19: The Potential Role of Extracorporeal Membrane Oxygenation [published online ahead of print, 2020 Feb 19]. JAMA. 2020;10.1001/jama.2020.2342. doi:10.1001/jama.2020.2342 [pubmed]
- Maharaj, R. King’s Critical Care – Evidence Summary Clinical Management of COVID-19. King’s College Hospital. https://www.nwpgmd.nhs.uk/sites/default/files/KCC%20COVID19%20Evidence%20Summary.pdf (26/3/2020) [No pubmed available]
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi:10.1016/S0140-6736(20)30628-0 [pubmed]
- Menter T, Haslbauer JD, Nienhold R, et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction [published online ahead of print, 2020 May 4]. Histopathology. 2020;10.1111/his.14134. doi:10.1111/his.14134 [pubmed]
- Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19) [published online ahead of print, 2020 May 1]. JAMA Cardiol. 2020;e201834. doi:10.1001/jamacardio.2020.1834 [pubmed]
- Mizumoto K, Chowell G. Estimating Risk for Death from 2019 Novel Coronavirus Disease, China, January-February 2020 [published online ahead of print, 2020 Mar 13]. Emerg Infect Dis. 2020;26(6):10.3201/eid2606.200233. doi:10.3201/eid2606.200233 [pubmed]
- Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi:10.2807/1560-7917.ES.2020.25.10.2000180 [pubmed]
- Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection [published online ahead of print, 2020 Mar 30]. Med Mal Infect. 2020;S0399-077X(20)30085-8. doi:10.1016/j.medmal.2020.03.006 [pubmed]
- Morawska L, Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality [published online ahead of print, 2020 Apr 10]. Environ Int. 2020;139:105730. doi:10.1016/j.envint.2020.105730. [pubmed]
- Moriguchi T, Harii N, Goto J, et al. A first Case of Meningitis/Encephalitis associated with SARS-Coronavirus-2 [published online ahead of print, 2020 Apr 3]. Int J Infect Dis. 2020;S1201-9712(20)30195-8. doi:10.1016/j.ijid.2020.03.062 [pubmed]
- Nishiura H, Kobayashi T, Suzuki A, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) [published online ahead of print, 2020 Mar 13]. Int J Infect Dis. 2020;S1201-9712(20)30139-9. doi:10.1016/j.ijid.2020.03.020 [pubmed]
- Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy [published online ahead of print, 2020 Mar 23]. JAMA. 2020 [pubmed]
- Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235–250. doi:10.1016/j.jhin.2015.08.027 [pubmed]
- Patel A, Jernigan DB; 2019-nCoV CDC Response Team. Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak – United States, December 31, 2019-February 4, 2020 [published correction appears in MMWR Morb Mortal Wkly Rep. 2020 Feb 14;69(6):173]. MMWR Morb Mortal Wkly Rep. 2020;69(5):140–146. [pubmed]
- Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features [published online ahead of print, 2020 Mar 31]. Radiology. 2020;201187. doi:10.1148/radiol.2020201187 [pubmed]
- Qiao J. What are the risks of COVID-19 infection in pregnant women?. Lancet. 2020;395(10226):760–762. doi:10.1016/S0140-6736(20)30365-2 [pubmed]
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China [published online ahead of print, 2020 Mar 3]. Intensive Care Med. 2020;1–3. doi:10.1007/s00134-020-05991-x [pubmed]
- Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases [published online ahead of print, 2020 Mar 20]. Lancet Respir Med. 2020;S2213-2600(20)30121-1. [pubmed]
- Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus Disease 2019 (COVID-19) and Pregnancy: What obstetricians need to know [published online ahead of print, 2020 Feb 24]. Am J Obstet Gynecol. 2020;. doi:10.1016/j.ajog.2020.02.017 [pubmed]
- Rodrigues JCL, Hare SS, Edey A, et al. An update on COVID-19 for the radiologist – A British society of Thoracic Imaging statement. Clin Radiol. 2020;75(5):323–325. doi:10.1016/j.crad.2020.03.003 [pubmed]
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis [published online ahead of print, 2020 Mar 13]. Travel Med Infect Dis. 2020;101623. doi:10.1016/j.tmaid.2020.101623 [pubmed]
- Rosenbaum L. The Untold Toll – The Pandemic’s Effects on Patients without Covid-19 [published online ahead of print, 2020 Apr 17]. N Engl J Med. 2020;10.1056/NEJMms2009984. doi:10.1056/NEJMms2009984 [pubmed]
- Rosenberg ES, Dufort EM, Udo T, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State [published online ahead of print, 2020 May 11]. JAMA. 2020;e208630. doi:10.1001/jama.2020.8630 [pubmed]
- Roy CJ, Milton DK. Airborne transmission of communicable infection–the elusive pathway. N Engl J Med. 2004;350(17):1710–1712. doi:10.1056/NEJMp048051 [pubmed]
- Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients [published online ahead of print, 2020 Mar 14]. AJR Am J Roentgenol. 2020;1–7. doi:10.2214/AJR.20.23034 [pubmed]
- Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi:10.1016/S1473-3099(20)30086-4 [pubmed]
- Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China [published online ahead of print, 2020 Mar 25]. JAMA Cardiol. 2020;e200950. doi:10.1001/jamacardio.2020.0950 [pubmed]
- Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr Opin Infect Dis. 2019;32(4):372–379. [pubmed]
- Sibthrope, C. “Newborn baby becomes ‘world’s youngest’ COVID-19 Victim. Sky News. 1/4/2020. Retrieved 5/4/2020. https://news.sky.com/story/coronavirus-newborn-baby-becomes-worlds-youngest-covid-19-victim-11967230
- Shao F, Xu S, Ma X, Xu Z, Lyu J, Ng M, Cui H, Yu C, Zhang Q, Sun P, Tang Z, In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China, Resuscitation (2020), doi: https://doi.org/10.1016/j.resuscitation.2020.04.005
- Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405‐407. doi:10.1016/j.healun.2020.03.012 [pubmed]
- Silverstein WK, Stroud L, Cleghorn GE, Leis JA. First imported case of 2019 novel coronavirus in Canada, presenting as mild pneumonia [published correction appears in Lancet. 2020 Feb 29;395(10225):e41]. Lancet. 2020;395(10225):734. doi:10.1016/S0140-6736(20)30370-6 [pubmed]
- Singh S, Chowdhry M, Chatterjee A, Khan A. Gender-Based Disparities in COVID-19: Clinical Characteristics and Propensity-matched Analysis of Outcomes. medRxiv 2020.04.24.20079046; doi:10.1101/2020.04.24.20079046 [no pubmed]
- Sit THC, Brackman CJ, Ip SM, et al. Infection of dogs with SARS-CoV-2 [published online ahead of print, 2020 May 14]. Nature. 2020;10.1038/s41586-020-2334-5. doi:10.1038/s41586-020-2334-5 [pubmed]
- Soldati G, Smargiassi A, Inchingolo R, et al. Is there a role for lung ultrasound during the COVID-19 pandemic? [published online ahead of print, 2020 Mar 20]. J Ultrasound Med. 2020;10.1002/jum.15284. doi:10.1002/jum.15284 [pubmed]
- Solaimanzadeh I. Acetazolamide, Nifedipine and Phosphodiesterase Inhibitors: Rationale for Their Utilization as Adjunctive Countermeasures in the Treatment of Coronavirus Disease 2019 (COVID-19). Cureus. 2020;12(3):e7343. [pubmed]
- Tang A, Tong ZD, Wang HL, et al. Detection of Novel Coronavirus by RT-PCR in Stool Specimen from Asymptomatic Child, China [published online ahead of print, 2020 Jun 17]. Emerg Infect Dis. 2020;26(6):10.3201/eid2606.200301. doi:10.3201/eid2606.200301 [pubmed]
- Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy [published online ahead of print, 2020 Mar 27]. J Thromb Haemost. 2020;10.1111/jth.14817. doi:10.1111/jth.14817 [pubmed]
- Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. Published 2020 May 14. doi:10.1136/bmj.m1849 [pubmed]
- Thachil J. The versatile heparin in COVID-19 [published online ahead of print, 2020 Apr 2]. J Thromb Haemost. 2020;10.1111/jth.14821. doi:10.1111/jth.14821 [pubmed]
- Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré Syndrome Associated with SARS-CoV-2 [published online ahead of print, 2020 Apr 17]. N Engl J Med. 2020;NEJMc2009191. doi:10.1056/NEJMc2009191 [pubmed]
- Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19 [published online ahead of print, 2020 Mar 30]. N Engl J Med. 2020;NEJMsr2005760. doi:10.1056/NEJMsr2005760 [pubmed]
- Valk SJ, Piechotta V, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;5:CD013600. Published 2020 May 14. doi:10.1002/14651858.CD013600 [pubmed]
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1 [published online ahead of print, 2020 Mar 17]. N Engl J Med. 2020;10.1056/NEJMc2004973. doi:10.1056/NEJMc2004973 [pubmed]
- Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein [published online ahead of print, 2020 Mar 6]. Cell. 2020;S0092-8674(20)30262-2. doi:10.1016/j.cell.2020.02.058 [pubmed]
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. [pubmed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China [published online ahead of print, 2020 Feb 7]. JAMA. 2020;e201585. [pubmed]
- Wang J, Hajizadeh N, Moore EE, et al. Tissue Plasminogen Activator (tPA) Treatment for COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS): A Case Series [published online ahead of print, 2020 Apr 8]. J Thromb Haemost. 2020;10.1111/jth.14828. doi:10.1111/jth.14828 [pubmed]
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens [published online ahead of print, 2020 Mar 11]. JAMA. 2020;e203786. [pubmed]
- Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569‐1578. doi:10.1016/S0140-6736(20)31022-9. [pubmed]
- Wakam GK, Montgomery JR, Biesterveld BE, Brown CS. Not Dying Alone – Modern Compassionate Care in the Covid-19 Pandemic [published online ahead of print, 2020 Apr 14]. N Engl J Med. 2020;10.1056/NEJMp2007781. doi:10.1056/NEJMp2007781 [pubmed]
- Weinkove R, McQuilten ZK, Adler J, et al. Managing haematology and oncology patients during the COVID-19 pandemic: interim consensus guidance [published online ahead of print, 2020 May 13]. Med J Aust. 2020;10.5694/mja2.50607. doi:10.5694/mja2.50607 [pubmed]
- Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19 [published online ahead of print, 2020 May 6]. Ann Intern Med. 2020;10.7326/M20-2003. doi:10.7326/M20-2003 [pubmed]
- Wilson N, Kvalsvig A, Barnard LT, Baker MG. Case-Fatality Risk Estimates for COVID-19 Calculated by Using a Lag Time for Fatality [published online ahead of print, 2020 Mar 13]. Emerg Infect Dis. 2020;26(6):10.3201/eid2606.200320. doi:10.3201/eid2606.200320 [pubmed]
- Wong HYF, Lam HYS, Fong AH, et al. Frequency and Distribution of Chest Radiographic Findings in COVID-19 Positive Patients [published online ahead of print, 2019 Mar 27]. Radiology. 2019;201160. doi:10.1148/radiol.2020201160 [pubmed]
- World Health Organization. Advice on the use of point-of-care immunodiagnostic tests for COVID-19, April 8 2020. https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19 (Accessed on 27/4/2020). [No Pubmed Available]
- World Health Organisation: Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. Version 1.2. 13/3/2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.Pdf (last accessed 29/3/2020) [No Pubmed Available]
- Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi:10.1126/science.abb2507 [pubmed]
- Wu X, Cai Y, Huang X, et al. Co-infection with SARS-CoV-2 and Influenza A Virus in Patient with Pneumonia, China [published online ahead of print, 2020 Mar 11]. Emerg Infect Dis. 2020;26(6):10.3201/eid2606.200299. [pubmed]
- Wu Y, Wang T, Guo C, et al. Plasminogen improves lung lesions and hypoxemia in patients with COVID-19 [published online ahead of print, 2020 Apr 10]. QJM. 2020;hcaa121. doi:10.1093/qjmed/hcaa121 [pubmed]
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention [published online ahead of print, 2020 Feb 24]. JAMA. 2020;10.1001/jama.2020.2648. doi:10.1001/jama.2020.2648 [pubmed]
- Lu X, Zhang L, Du H, et al. SARS-CoV-2 Infection in Children [published online ahead of print, 2020 Mar 18]. N Engl J Med. 2020;10.1056/NEJMc2005073. doi:10.1056/NEJMc2005073 [pubmed]
- Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020 [pubmed]
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published online ahead of print, 2020 Feb 24] [published correction appears in Lancet Respir Med. 2020 Feb 28;:]. Lancet Respir Med. 2020;S2213-2600(20)30079-5. doi:10.1016/S2213-2600(20)30079-5 [pubmed]
- Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review [published online ahead of print, 2020 Mar 19]. Eur Radiol. 2020;10.1007/s00330-020-06801-0. doi:10.1007/s00330-020-06801-0 [pubmed]
- Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis [published online ahead of print, 2020 Apr 10]. J Infect. 2020;S0163-4453(20)30191-2. doi:10.1016/j.jinf.2020.03.062 [pubmed]
- Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible?. Lancet Gastroenterol Hepatol. 2020;5(4):335–337. doi:10.1016/S2468-1253(20)30048-0 [pubmed]
- Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2 [published online ahead of print, 2020 Apr 3]. J Thromb Thrombolysis. 2020;10.1007/s11239-020-02105-8. doi:10.1007/s11239-020-02105-8 [pubmed]
- Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore [published online ahead of print, 2020 Mar 3]. JAMA. 2020;e203204. doi:10.1001/jama.2020.3204 [pubmed]
- Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020;10:40. Published 2020 Mar 16. doi:10.1186/s13578-020-00404-4 [pubmed]
- Zhang C, Huang S, Zheng F, Dai Y. Controversial treatments: an updated understanding of the Coronavirus Disease 2019 [published online ahead of print, 2020 Mar 26]. J Med Virol. 2020;10.1002/jmv.25788. doi:10.1002/jmv.25788 [pubmed]
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038]. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3 [pubmed]
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7 [pubmed]
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi:10.21037/tp.2020.02.06 [pubmed]
- Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi:10.1056/NEJMoa2001017 [pubmed]
- Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online ahead of print, 2020 Mar 28]. Clin Infect Dis. 2020;ciaa344. doi:10.1093/cid/ciaa344 [pubmed]
- Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study [published online ahead of print, 2020 Mar 3]. AJR Am J Roentgenol. 2020;1–6. doi:10.2214/AJR.20.22976 [pubmed]
- Ziehr DR, Alladina J, Petri CR, et al. Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study [published online ahead of print, 2020 Apr 29]. Am J Respir Crit Care Med. 2020;10.1164/rccm.202004-1163LE. doi:10.1164/rccm.202004-1163LE [pubmed]
FOAM and web resources
- ACEM COVID-19 (Resources from the Alfred ICU, including guidelines and evidence reviews)
- ANZICS Coronavirus Resources
- Australian Department of Health – Coronavirus (COVID-19) resources
- COVID-19 Pandemic, The Charts of Our World in Data
- Emergency Intubation of COVID-19 patient (video) (INTENSIVE)
- EMCrit Internet Book of Critical Care (IBCC) – COVID-19
- INTENSIVE – COVID19 (Alfred ICU COVID19 resources)
- Australian National COVID-19 Clinical Evidence Taskforce Guidelines
- WHO COVID-19 Situation Report
SARS-CoV-2
novel coronavirus of COVID-19

updated and comprehensive. excellent
Thanks Anurag
Cheers
Chris
Hi thanks for all the excellent info.
I’m a rural emerg doc 40 mins away from the closest ICU.
There seems to be a lot in the FOAMed world this week about not necessarily jumping to intubate our COVID patients (perhaps trying HFNC>NIPPV +/- proning).
Any recommendations for transporting these patients safely (for both the patient and the ems crew). Any thoughts on how to transport this subset of patients with high oxygen requirements safely and stave off intubation? Is it even an option to transport on CPAP?
Stay well!
Hi Bradley
Transport of COVID-19 patients, even within hospital, can be challenging as it involves balancing the risks of deterioration with the risks of virus transmission, and trying to keep things simple.
Early intubation may still be required for safe transfer.
Adequate oxygen supply can be a major issue for HFNC.
CPAP on a transport ventilator is feasible but should be part of a standard operating procedure.
Increasingly, awake proning and proning during transport is being performed around the world – though I have no personal experience of this. Similarly, I am aware of inhaled NO having been used for transports by experts.
Any retrieval medicine experts, wish to comment on this?
Cheers
Chris
In the “prognosis” subsection, under the bullet point that reads “fatality estimates may also be influenced by”, please add a bullet point that says something to the effect of “lack of widespread antibody testing.” Because we don’t know how many people have already been infected and recovered and we don’t know how long the virus has been circulating, the denominator when estimating the fatality rates is most likely much larger, and therefore, the fatality rates that are being reported based on number of cases from positive tests or assumed positive are inflated.
Thanks Adrian, this has been clarified
Cheers
Chris
[…] mer traditionell sammanställning här, bra om referenser […]
CoViD impairs hypoxic pulmonary vasoconstriction hypothesis:
Consider these interventions to reduce intra-pulmonary shunting:
(60 years of physiology research)
Mild hyperthermia
L-lactic acid infusion, buffered.
TASK-1 channel blocker infusion eg doxapram, almitrine.
Desferrioxamine infusion, Mn.
Anandamide infusion
Asymmetric dimethylarginine (AMDA) infusion
Arginase inhibitor (nor-NOHA) infusion
Angiotensin II infusion
Beta-2 agonist infusion
Avoid vasodilators (CCB)
Position CoViD lobules upper.
Also note that hypoxia and acute phase reactants increase thromboxane production. Thromboxane maybe responsible for some of the pathophysiology of COVID-19.