Humongous HIV Extravaganza
aka Tropical Travel Trouble 009
The diagnosis of HIV is no longer fatal and the term AIDS is becoming less frequent. In many countries, people with HIV are living longer than those with diabetes. This post will hopefully teach the basics of a complex disease and demystify some of the potential diseases you need to consider in those who are severely immunosuppressed.
While trying to be comprehensive this post can not be exhaustive (as you can imagine any patient with a low CD4 count – all bets are off) and aims to focus on the key learning points surrounding HIV.
Q1. What is HIV?
Answer and interpretation
Human immunodeficiency virus (HIV) is caused by a lentivirus (subgroup of retrovirus) that infects and destroys immune cells expressing CD4 (T-helper cells, macrophages and dendritic cells).
There are two viruses, HIV-1 and HIV-2. HIV-1 is more common and aggressive than HIV-2. HIV-2 is largely confined to West Africa and to areas with West African immigrants. Co-infection can occur as infection with one does not protect against the other. Transmission and clinical presentation are similar except that HIV-2 takes several years longer than HIV-1 to cause significant immunosuppression (Kaposi’s sarcoma is generally not seen in HIV-2). It is mentioned only to emphasise that testing kits are required to test for both strains. While clinical management is generally not altered, non-nucleoside reverse transcriptase inhibitors have no activity in HIV-2 and therefore are avoided (see drugs below).
Q2. How does HIV infect our cells and replicate?
Answer and interpretation
Below is a wordy summary of how HIV infects our cells, you may prefer to jump to the videos and then read the content.
1. HIV expresses a receptor GP120 and GP41 which attaches to cells that express CD4 and a co-receptor molecule (CCR5 or CXCR4) on the cell surface. Once bound, the HIV molecule unfolds the GP41 and it fuses the two cell membranes.
2. The capsid enters the cell and is dissolved releasing 2 viral RNA strands and 3 essential replication enzymes (Integrase, Reverse Transcriptase and Protease)
3. Viral reverse transcriptase starts the reverse transcription (good name for it). It has two catalytic domains: ribonuclease H active site and a polymerase active site. The polymerase site transcribes the viral RNA into a RNA/DNA double helix.Ribonuclease H breaks down the RNA leaving a single strand of DNA. This strand of DNA takes a second pass through the polymerase active site leading to a new DNA double helix, which is then inserted into the host cell’s DNA by integrase.
4. Viral messenger RNA is then transcribed from the viral DNA and taken to ribosomes in the cytoplasm. The mRNA is read by the ribosomes and creates viral amino acid strands. These strands along with 2 viral RNAs are released from the cell as an immature HIV. Protease splices the long amino acid strands in this immature cell to the exact proteins the HIV needs. The cell is now a mature virus ready to infect another cell.
The following videos are very helpful for revision on reverse transcription and the important concepts of how HIV replicates. You will need to understand this to understand how the drugs work. If you are fairly confident then just jump to video 3 for a re-cap.
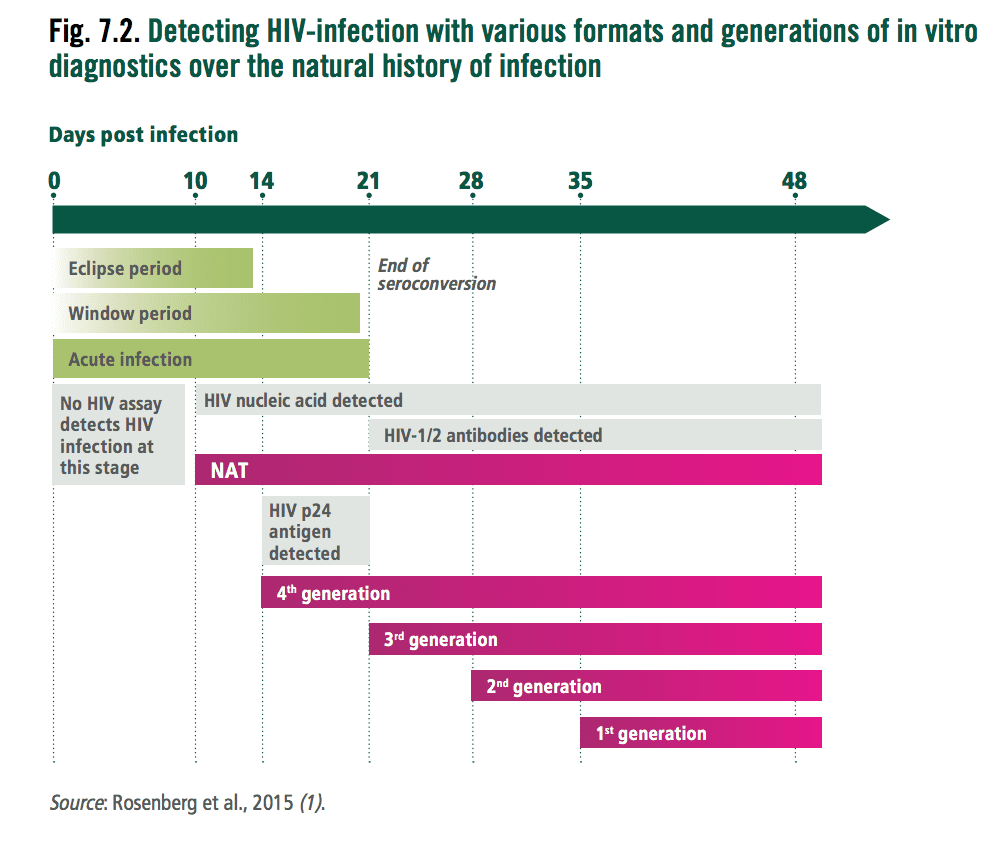
Q3. How do you diagnose HIV and what is the earliest time you can test for it?
Answer and interpretation
- HIV antibodies, antigen (p24) or Viral DNA/RNA via PCR. Commonly the rapid tests look for HIV antibodies and antigen p24.
- Most testing kits (4th generation) work from day 14 when antibodies or the p24 antigencan be detected
- In cases of acute HIV, nucleic acid amplification tests will turn positive approximately 8-10 days after exposure
- Viral DNA or RNA detection is discussed in the paediatric section and can work from day 10 post infection.
For a period of 10 days known as the eclipse period no currently available serological or virological assays can detect HIV. The end of the eclipse phase is marked by the appearance of HIV RNA or DNA detectable by nucleic acid testing and then HIV p24 antigen by immunoassay.
The period prior to the detection of HIV 1 or 2 antibodies is the “acute infection” period. During this time the HIV virus numbers climb dramatically and it is associated with higher infectivity and rates of transmission.
The window period is the time between HIV infection and the earliest time a serological assay can detect HIV 1 or 2 antibodies. It is important that you know the window period of the test you are using. If the test is used in the ‘window period’ a repeat test is required outside this period. The duration of the window period depends on 3 factors:
- The genetics of the virus
- The genetics and immunocompetence of the host.
- What exactly the assay detects (antigen or antibodies).
Beyond the initial test (if used correctly and the result is positive), a repeat test is required to confirm the diagnosis, in resource poor settings typically a different brand of a 4th generation test is used. In other settings a HIV 1 / HIV 2 immunoassay or viral detection. Western blot is being phased out due to false negative pick up on HIV 2 and the time delay to results.
Special consideration is required for children under 18 months (see paediatric section).
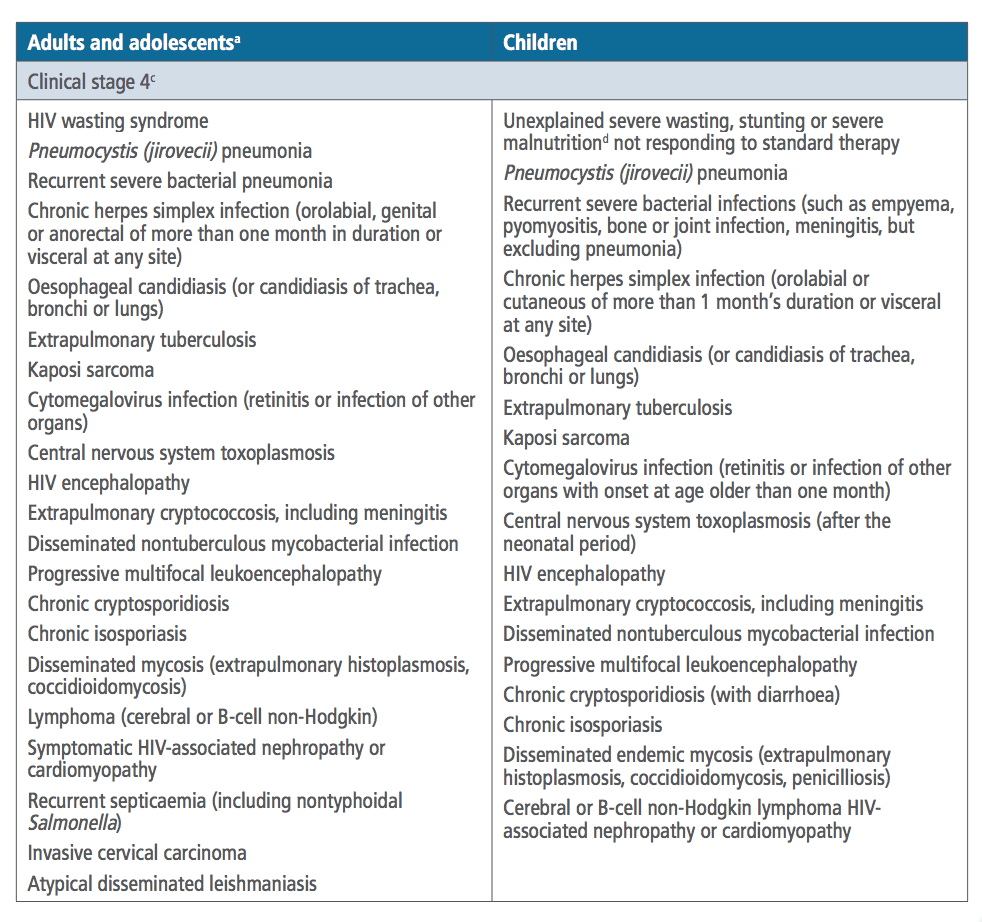
Q4. What is the WHO clinical staging system for HIV when CD4 testing is limited?
Answer and interpretation
The CDC defines AIDs as a CD4 count <200 or an AIDs defining condition. In some countries it is impossible to get a CD4 count. The WHO system is a clinical staging system based on history and presenting diagnoses, it is not only useful for initial diagnosis but also to monitor for treatment failure in resource poor settings (i.e. if the patient develops a disease in the next stage while ‘on treatment’).
- Stage 1 = Asymptomatic or persistent generalised lymphadenopathy
- Stage 2 = Mild opportunistic infection or weight loss
- Stage 3 = Serious or persistent opportunistic infections / severe weight loss / Pulmonary TB (+lymph node TB in children) / Cytopenias
- Stage 4 = AIDs defining conditions
Q5. What is the difference between viral load and CD4 count?
Answer and interpretation
Viral load:
- Measure of plasma RNA or DNA per mL.
- RNA reflects non-integrated viral nucleic acid and DNA reflects viral nucleic acid already integrated into the human genome. Most reference to viral load reflect the pre-negrated RNA levels.
- High viral load in primary infection (at seroconversion) then reaches a “set point”.
- Equates to disease progression and infectiousness to others.
- Aim of antiretroviral therapy (ART) is to achieve and maintain full virological suppression (undetectable = untransmissionable).
- VL is important to monitor treatment response.
CD4 Count:
- Measure of immunological damage and risk of opportunistic disease.
- CD4 levels can vary during the natural history of infection. Levels can be low during primary infection and then recover spontaneously prior to a slow decrease towards 0 over 5-10 years.
- Poorer prognosis with lower nadir CD4 count and longer time spent with low CD4 count.
- Immune reconstitution on ART (due to reduced VL) reduces risk of opportunistic disease.
- Important in determining disease stage.
- CD4 declines 0-159 cells per year in those with a viral load of 3-5.5 log copies/mL.
The diagram below illustrates the above viral load and CD4 count interaction.
The patient becomes infected, viral load goes up and CD4 count drops to a set point and nadir respectively. The patient experiences an acute HIV syndrome before a period of clinical latency where there is initially a drop in the viral load and increase in the CD4 count. The viral load gradually increases, the CD4 count gradually declines until there are opportunistic infections resulting in death.
Q6. What are the anti-retrovirals available?
Answer and interpretation
If you are like me then the following list of drug names and 3 letter abbreviations is impossible to remember, start with knowing most regimens are 3 drugs from at least 2 different major categories.
The original players were the Nukes and the Non-Nukes (nukes = nucleoside inhibitors) which stop the reverse transcriptase. As you can imagine (if you’ve watched the videos above) it would be nice to stop the protease and integrase from working, hence the inhibitors for these and finally it would be great to stop the HIV virus from binding in the first place (Fusion and attachment inhibitors).
In bold are the commonly used drugs:
Nucleoside reverse transcriptase inhibitors (NRTI) – aka the nukes, act as false nucleosides and cause chain termination.
- Abacavir (ABC)
- Emtricitabine (FTC) – also active against Hep B
- Lamivudine (3TC) – also active against Hep B
- Tenofovir disaproxil (TDF) or tenofovir alafenamide (TAF) – also active against Hep B
- Zidovudine (AZT)
- Didanosine (DDI)
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) – aka the non-nukes. Work by inhibiting the reverse transcriptase enzyme through other mechanisms of inhibition unrelated to false nucleosides.
- Efavirenz (EFV)
- Nevirapine (NVP)
- Rilpivirine (RPV)
- Etravirine (ETR)
Protease inhibitors. Blocks the protease enzyme responsible for reassembly of the virus after replication. PI’s often require a pharmacological booster – Ritonavir (RIT) or Cobicistat is frequently used, The small “r” depicted as a ‘r’ on the end of the 3 letter abbreviations for drugs used can signal ritonavir (i.e. DRV/r)
- Atazanavir (ATV)
- Darunavir (DRV)
- Fos-Amprenavir
- Lopinavir (LPV)
- Saquinavir (SAQ)
- Tipranavir (TPV)
Integrase Inhibitors. Blocks the viral DNA integrating into the host’s DNA.
- Raltegravir (RAL)
- Elvitegravir (EVG)
- Dolutegravir (DTG)
- Bictegravir (BIC)
Fusion inhibitors:
Attachment inhibitors (CCR5):
Fixed dose combinations (not a complete list):
Dual:
- Combivir AZT + 3TC
- Kivexa ABC + 3TC
- Truvada TDF + FTC
- Descovv TAF + FTC
- Kaletra Lop/r
- Prezista (DRN/r)
- Prezcobix (DRN/c)
- Juluca (DTG/RPV)
Triple:
- Trizivir ABC+3TC+AZT
- Trioday TDF+3TC+EFV
- Viraday TDF+3TC+EFV
- Atripla TDF+FTC+EFV
- Eviplera or Complera TDF+FTC+RPV
- Odefsey TAF + FTC + RPV
- Stribld TVD+FTC+EVG/c
- Genvova TAF + FTC + EVG/c
WHO recommends 2 nukes and one non-nuke: 1st line recommendation = TLE (TDF+3TC+EFV) or Trioday (but soon to be changing due to the integrase inhibitors being so well tolerated – watch this space). But in most low middle income settings just remember TLE, TLE, TLE, except in children, TDF (Tenofovir) is replaced with ABC or TAF due to potential bone growth inhibition. >3 years of age ABC + 3TC + EFV (ALE) or <3 years of age ABC + 3TC + LPV/r (ALL). All prescribers should be aware of the national HIV guidelines for their nation of practice as many will vary from country to country based on availability/cost/distribution.
Q7. What are the common side effects of ART?
Answer and interpretation
NRTIs (nukes)
- TDF – proximal renal tubulopathy, Fanconi’s syndrome, osteoporosis.
- ABC – SJS, 8% of patients, may increase risks of cardiovascular disease but literature is contradictory.
- AZT, DDI – lipodystrophy, lactic acidosis, pancreatitis, peripheral neuropathy, macrocytic anaemia, liver cirrhosis.
NNRTIs (non-nukes)
- Efavirenz – CNS toxicities include worsening depression and abnormal dreams. Use with caution in patients with pre-existing psychiatric diagnoses.
- Nevirapine – rash 4% (mild to severe SJS and TEN), drug induced hepatitis in up to 10%.
- Etravirine – rash 4% (mild to severe SJS and TEN)
Protease inbititors (all end in ‘navir) commonly cause diarrhoea in the early generations but is improved in more modern regimens. Frequently will cause drug/drug interactions due to the presence of ritonavir or cobicistat which can boost other agents.
- Insulin resistance / diabetes
- Dyslipidaemia
- Lipodystrophy
- Jaundice and renal stones with Atazanavir
- Drug induced hepatitis
- Avascular necrosis
Integrase inhibitors – very good tolerability and fewer drug interactions.
Q8. When do you start ART?
Answer and interpretation
As soon as possible, preferably within the first 2 weeks of diagnosis, this includes: adults, children, pregnant and breastfeeding women.
It used to be when CD4 counts were <350 cells/mm3 but subsequent studies have showed benefit starting immediately.
Exceptions:
- TB meningitis and the risk of IRIS – between 4-8 weeks (see below for further details about IRIS).
- Cryptococal meningitis after 4 weeks of induction and consolidation treatment with amphotericin B containing regimens are the current recommendations, again for concerns of an IRIS type immune reactivation.
- If the patient is not ready. Ideally, ART should start as soon as possible; however, if there are concerns over adherence than prescribers may elect to withhold treatment to limit the development of antiviral resistance in the setting of intermittent dosing.
Q9. What baseline investigations would you do?
Answer and interpretation
In low middle income settings I was taught to get baseline bloods, check for pregnancy and HIV infection in the family (so far so good). Then for specific opportunistic infections think:
- 2 in the head (cryptococcal, toxoplasma),
- 2 in the chest (TB and PCP),
- 2 in the belly (Hep B and Hep C) and
- 2 down below (Syphilis and STI screen).
Opportunistic infections:
- Cryptococcal antigen
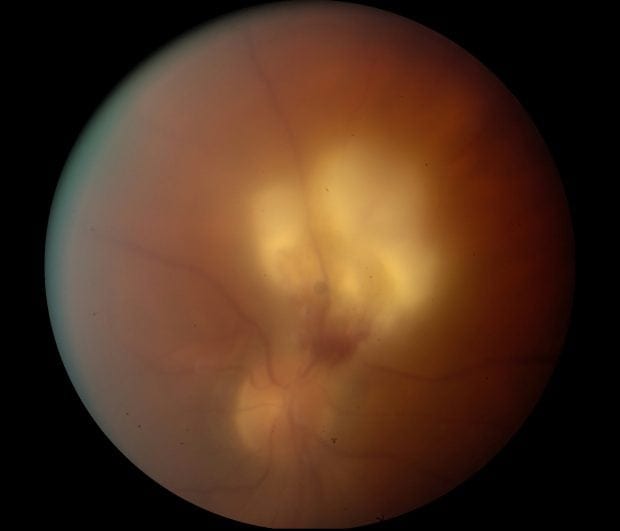
- Toxoplasma – look in the eyes
- TB – CXR + sputum, ask about the four key symptoms (current cough, night sweats, weight loss and fever).
- PCP – CXR
- Hep B sAg
- Hep C Antibody
- Syphilis
- STI screen (increases the risk of transmission)
Others:
- FBC
- U+Es
- LFTs
- Glucose
- Urine dipstick
- Pregnancy Test
- Partner and children testing
Other investigations because I have a big fancy lab (aka first world setting):
- HIV-1 plasma viral load
- HIV-1 drug resistance test
- CD4 count
- Hep A
- Measles/varicella antibodies (according to vaccination / infection history)
- Bone profile and fracture risk assessment
- Cardiovascular risk assessment
- Psychological assessment
- Test patients for parasitic infection if persistent eosinophilia on FBC and relevant travel history.
- HLA-B 57:01 if abacavir therapy is being considered
- Viral tropism test if a CCR5 inhibitor is being considered
- Women:
- Cervical cytology if not done in the past 12 months
- Rubella if child-bearing potential
Q10. Apart from ART what other treatments should you consider?
Answer and interpretation
Co-trimoxazole prophylaxis is recommended in adults (including pregnant women) with severe or advanced HIV clinical disease (WHO stage 3 or 4) and/or with a CD4 count <350 cells/mm3.
- Malaria and severe bacterial infections are highly prevalent, co-trimoxazole should be lifelong.
- Co-trimoxazole may be discontinued in adults (including pregnant women) with HIV who are clinically stable on ART, with evidence of immune recovery and viral suppression.
- It should also be given in all HIV patients with active TB.
- In children co-trimoxazole is continuous until aged 5 then lifelong if in a malaria/severe bacterial infection setting or can stop if stable (viral suppression).
Primary prophylaxis:
- Pneumocystosis / Cerebral toxoplasmosis, Isosporiasis and Malaria = Cotrimoxazole PO
- Adults: 800mg SMX + 160mg TMP once a day
- Children: 50mg SMX + 10mg TMP/kg once a day
The WHO recommends 6 months of Isoniazid Prophylaxis Treatment (IPT) should be offered to all patients living with HIV in nations with high TB endemicity.
- Patients should be screened for the 4 symptoms of: current cough, fever, weight loss or night sweats, if all negative and the addition of a chest X-ray the chances of active pulmonary TB are low and they should be offered IPT.
- If a tuberculin skin test is unknown or positive, IPT should be for 6 months (if TB can not be safely ruled out) or 36 months if active TB can be ruled out.
If you suspect active TB, a Gene Xpert MTB/RIF should be used instead of conventional microscopy. Culture and drug susceptibility testing should also be done if possible. Patients with CD4 counts <100 cells/mm3 should have a urinary LAM test as this has the greatest sensitivity.
TB patients with HIV should be treated with a rifampicin/rifabutin backbone as per standard guidelines. Use HIV drug interactions to refine your treatment regimen further.
Children more than 12 months who do not have any of the classic four symptoms should have 6 months of IPT (10 mg/kg). Under 12 months, only those with a TB contact and have a negative work-up for TB should get 6 months of IPT.
Q11. What defines treatment failure?
Answer and interpretation
Persistently detectable viral load exceeding 1000 copies/ml after at least 6 months of starting a new ART regimen:
- Requires two consecutive viral load measurements within a 3 month interval and…
- Adherence support between measurements.
If you suspect treatment failure seek expert advice, in general, never add a single agent to a failing regimen and often switching classes of ART is necessary. Some regimens recommended by WHO are listed below:
- Adult ART switch (if failed on TDF + 3TC (or FTC) = AZT + 3TC + LPV/r
- Paediatric ART switch:
- 1st line = 2NRTIs + Lop/r
- 2nd line:
- <3yrs: 2 NRTIs + RAL
- >3 years: 2 NRTIs + EFV or RAL
- If started on 2 NRTIs + EFV change to 2 NRTIs + ATV/r or Lop/r
Q12. What about resistance?
Answer and interpretation
Clinical resistance occurs in the context of non-suppressive ART exposure.
Increasing duration on a failing regimen leads to accumulation of resistance.
Once drug pressure removed, resistant mutants are outgrown by fitter wild-type virus (i.e. the original virus)
- Resistant mutants persist at low-level: archived in latent memory T cells.
- Resistance only detectable by standard tests if the patient is taking ART at the time of testing (so the original virus is suppressed allowing for the resistant strain to accumulate).
Viral load is the best to monitor treatment failure.
Q13. What is TASP?
Answer and interpretation
Treatment as prevention – getting the viral load to undetectable prevents transmission
HIV treatment can reduce HIV transmission by 96%.
Q14. When to give PEP?
Answer and interpretation
ART to reduce the likelihood of HIV infection either after occupation exposure or sexual exposure.
PEP is not routinely recommend after sex with an HIV positive source who is on ART with a confirmed and sustained (>6 months) undetectable plasma HIV viral load (<200c/ml).
PEP can be offered up to 72 hours post exposure but ideally the sooner the better with the best results from 2 hours.
First line regimen in the UK is Truvada (Tenofovir (TDF) and Emtricitabine (FTC))and Raltegravir for 28 days with an infectious disease follow up during this time. This regimen was picked due to safety and tolerability over other combinations.
If further risk occurs during the last two days of the PEP course, then PEP should be continued for 48 hours after the last high-risk exposure.
If more than 48 hours of PEP is missed it should be discontinued.
Follow-up HIV testing is recommended 8-12 weeks after exposure.
PEP is recommended when the risk of HIV transmission is >1/1000. It should be considered when the risk of transmission is between 1/1000 and 1/10,000.
UK national guideline risk table:
Exact PEP regimens and risk tables may differ depending on your country or local populations. See the Australian guidelines here. The main difference is the PEP regimens and that in those where the HIV status is unknown a 2 drug regimen is considered.
Pregnancy is not a contraindication for PEP but all cases should be discussed with infectious diseases as these drugs are not licensed for pregnancy and a thorough risk assessment will need to be made.
Need to consider Hep B status/immunization and STI screening when indicated.
Q15. What about PrEP?
Answer and interpretation
The use of antiretrovirals in HIV-uninfected people to block the acquisition of HIV infection.
The PrEP regimen of oral tenofovir-emtricitabine (TDF-FTC) taken daily or on-demand prior to a potential risk exposure.
Efficacy:
- iPrex, phase 3 randomised double bind = 44% reduction in HIV transmission.
- PROUD, phase 3 open label RCT = 86% reduction in HIV transmission.
- IPERGAY open label demonstrated a 97% reduction in HIV transmission risk compared to the placebo are of the IPERGAY randomised phase.
- Efficacy is strongly related to compliance as the majority of seroconversion occurred in those who were non-compliant or had delayed administration of PrEP.
Safety:
- RCTs have shown good safety data for daily and on-demand oral TDF-FTC as PreP in men who have sex with men.
- TDF-FTC was associated with mild, non-progressive and reversible reduction in creatine clearance.
- Age >40 years and having a CrCl <90ml/min at baseline prior to starting PrPE were independently associated with a (small) risk of Cr Cl falling to <60 ml/min.
Dosing recommendations:
- If the risk of HIV acquisition is through anal sex, PrEP can be started with a double dose of TDF-FTC taken 2-24 hours before sex and continued daily until 48 hours after the last sexual risk.
- If PrEP for anal sex has been interrupted and it is less than 7 days since the last TDF-FTC dose then PrEP can be re-started with a single dose of TDF-FTC.
- Also for anal sex, four or more daily doses each week will give good protection, especially after the first week.
- If the risk of HIV acquisition is through vaginal sex, PrEP should be started as a daily regimen 7 days ahead of the likely risk and continued daily for 7 days after the last sexual risk.
- The differences in drug regimen lead-in times is because it takes longer for the drug levels to peak in vaginal tract than in anal canal.
PrEP resources:
- I want PrEP now
- Prepster.info
- Pocket PrEP: UK guide
- BHIVA PrEP guidelines 2017
Q16. TB is the most common opportunistic infection in HIV patients and cause of death. How do you diagnose TB in this patient cohort?
Answer and interpretation
Quick facts:
- Incidence of active TB is 21-37 fold higher in HIV+ve.
- Risk of developing TB is 5-15%/year (0.1% in HIV -ve individuals).
- Increased risk with falling CD4 count.
- ART reduces mortality by 64-95%.
Adults and adolescents living with HIV should be screened for TB. If they do not report any current cough, fever, weight loss or night sweats they are unlikely to have active TB and should be offered preventative therapy (isoniazid for 6 months). CXR may be offered as an additional step to check for active disease (same protocol for HIV negative patients).
Inconsistency of TST and IGRA in HIV+ve patients, although IGRA is the current UK recommendation for detecting latent TB. Neither are recommended for active TB diagnosis.
Altered clinical presentation of TB, increase in smear negative pulmonary disease and increase in extra-pulmonary/disseminated forms.
For active TB, first line testing is with a GeneXpert MTB/RIF, however other settings advocate still performing microscopy. Culture and drug sensitivities should also be performed.
Q17. When should you start ARTs in the TB patient?
Answer and interpretation
ART should be started in all TB patients living with HIV regardless of their CD4 count.
TB treatment should be initiated first, followed by ART as soon as possible in the first 8 weeks of treatment. Profound immunosuppression with CD4 counts <50 cells/mm3 should receive ART within the first 2 weeks of initiating TB treatment. 8 trials had high quality of evidence to show starting ART before 8 weeks improved mortality. 4 of the trials also showed the benefit was higher when started within 2 weeks.
TB regimens cause drug interaction with ARTs for example, rifamycins induce hepatic CYP3A4 enzymes that can accelerate metabolism of protease inhibitors and some nonnucleoside reverse transcriptase inhibitors (NNRTIs). Despite the complexity of managing drug interactions, rifamycins are integral to the success of TB therapy and should not be substituted with other antituberculosis medications based on concerns regarding drug interactions alone.
See HIV drug interactions to simplify a regimen (and speak to a specialist).
Q18. What about TB meningitis and IRIS, when should you start ARTs?
Answer and interpretation
TBM-associated immune reconstitution inflammatory syndrome (TBM-IRIS), which comprises exaggerated inflammation in response to M.Tuberculosis.
IRIS describes a collection of inflammatory disorders associated with paradoxical worsening of pre-existing infectious processes following the initiation of antiretroviral therapy in HIV +ve individuals. The immune system wakes up with ART and goes into overdrive. If your TB is a cold abscess on your back and you get IRIS, it doesn’t really matter if this swells or associated lymph nodes swell, but the same situation intracranially can potentially be life threatening – remember the Munro Kelly doctrine.
From one study it has been recommended that ART-naïve HIV-infected patients with CNS tuberculosis, initiation of ART should be delayed for the first eight weeks of antituberculous therapy, regardless of CD4 count. There was no difference in survival but reduced Grade 4 adverse events (co-morbidities resulting in immobility).
TB IRIS case definition.
Need a diagnosis of TB and an initial response to treatment prior to starting ARTs. 1 major and 2 minor:
- Major = new or enlarging LN, cold abscess or other focal tissue involvement. New or worsening radiological features of TB. New or worsening CNS TB. New or worsening serositis (pleural or pericardial effusion).
- Minor = new or worsening constitutional symptoms, respiratory symptoms, abdominal pain, hepatosplenomegaly, peritonitis.
- Alternative explanations for the deterioration can not be excluded.
Risk factors:
- Low CD4 count <50
- High HIV viral load
- ARVs started early <2 months after treatment of TB
- Rapid reduction in viral count
- Rapid rise in CD4 count
No treatment in most cases, no need to stop ARVs, continue TB treatment.
Symptomatic treatment, consider steroids and drainage of any abscess.
Q19. Your HIV patient has had diarrhoea for a month and can not swallow due to ordynophagia, what is your differential and treatment?
Answer and interpretation
Pre ART era a study from Zaire found 84% of patients with diarrhoea lasting over 1 month had HIV.
A large proportion of lymphocytes reside in the Gut Associated Lymphoid Tissue (GALT) and are destroyed by HIV infection.
Algorithm for dysphagia – treat for oesophageal candidiasis if no better treat for HSV, if no better arrange endoscopy/barium swallow.
Practically, prolonged diarrhoea and fever treat with ciprofloxacin/azithromycin for 3-5 days depending on known local resistance. If no response treat for amoebic dysentery with metronidazole 800mg TDS for 5-10 days. If no response look in the stool for ova and parasites or other causes of the diarrhoea. Can also add co-trimoxazole 960mg QDS for 7 days (treat isopoda, cyclosporine, cryptosporidium, bacterial infections) and then add albendazole 400mg BD for 21 days to treat microsporidia and enteric helminths.
50% of the time no cause is found.
Q20. Your next HIV patient has deranged LFTs, what is your differential?
Answer and interpretation
Viral: Viral hepatitis (HBV/HCV), HIV (often a co-infection).
- Can combine with tenofovir, lamivudine and emtricitabine. Well tolerated. Shouldn’t use lamivudine on its own as Hep B resistance can develop.
- HCV was complicated to treat but now…. pangenotypic agents exist and guidelines are currently in flux. Epiclusa (sofosbuvir and velpatasvir) for 12 weeks +/- ribavirin. Or Maviret (Glecaprevir and pibrentasvir) for 8-12 weeks. Interactions with NNRTI and PI’s but okay with integrates and NRTIs.
Mycobacterial: MTB complex and non-tuberculous mycobacteria.
Fungal: cryptococcus, histoplasmosis, candidiasis, PCP.
Parasitic: Liver flukes, cryptosporidium, micosporidium.
Malignancy: Lymphoma and Kaposi sarcoma.
Drugs: consider drug induced hepatitis if:
- AST/bili >5x ULN or 3.5x baseline.
- Coagulopathy, eosinophilia, rash and lactic acidosis.
Consider TB IRIS if:
- Evidence of IRIS in another system.
- New tender hepatomegaly.
- Predominantely cholestatic.
- USS shows diffusely abnormal liver texture, LN and a normal biliary tree.
- Stop treatment if LFTs >5x upper limit of normal without symptoms or >3x ULN if symptomatic (anorexia, nausea, abdo pain) or there is a significant bilirubin rise. Once bilirubin normalised or ALT/AST <2x ULN can reintroduce drugs. A holding pattern for drugs would be ethambutol and moxifloxacin or streptomycin.
- Can reintroduce all the drugs at once if mild or moderate, or one by one over 10 days.
Q21. If you saw an HIV patient for breathlessness, what would be in your differential?
Answer and interpretation
Bacterial:
- Strep pneumo, staphylococal, klebsiella.
- Non-typhoidal salmonellae, pseudomonas, haemophilis influenzae, mycoplasma pneumoniae.
- Rhodococcus equi, nocardia spp.
Mycobacteria (TB and non-TB)
Fungi:
- Pneumocystis jirovecii – exertion dyspnoea, dry cough, prolonged symptoms, CD4 count <200, LDH >600, hypoxia. Sub-saharan Africa studies showing rate between 10-30% in in-patients that are TB smear -ve.
- Cryptococcus neoformans, histoplasmosis capsulatum, penicillium marneffei, coccidiosis imitis, candida albicans.
- Aspergillus spp – possibly.
Viruses:
- Influenzae, adenovirus, rhinovirus, CMV….
Malginancy:
Pulmonary pathology by CD4 status:
- >500 = Bacterial pneumonia, TB, Influenza, Endemic mycosis.
- 200-500 = Recurrent bacterial pneumonia, pulmonary varicella zoster.
- 199-100 = Pneumocystis jiroveci penumonia, histoplasmosis, cryptococcosis, rhodeococcus, nocardia.
- <100 = CMV, MAI, fungal, herpes simplex …
Pneumonia antibiotics:
- Mild = Amoxicillin 500mg TDS PO for 7/7.
- Moderate = IV benzylpenicillin 2MU Q4hly +/- macrolide.
- Severe = IV benzylpenicillin + genatmicin 5mg/kg IV OD + macrolide.
TB – ART should start ASAP, ART my reduce mortality by 64-95%
Q22. You do a lumbar puncture on an HIV patient and get a predominance of lymphocytes, what are your differentials?
Answer and interpretation
Nervous system disorders among patients receiving ARTs >25%
Differential for lymphocytic CSF:
- Viral meningitis/encephalitis
- TBM
- Listeria
- Fungal meningitis – cryptococcus
- Partially treated bacterial meningitis
- Parameingeal infections (cerebral abscess)
- Syphilis
- HIV
- Autoimmune encephalitis
- Drugs – NSAIDs, antibiotics
- ADEM (acute demyelinating encephalomyelitis)
- MS
- Neoplastic/paraneoplastic
- Sarcoid
- Vasculaitis
- Autoimmune – SLE
Q23. You investigate the patient with a CT and find a ring enhancing lesion, what are your differentials?
Answer and interpretation
CT ring enhancing lesions – no appearance is pathognomonic. See radiopaedia for examples.
- The more lesions the more likely its toxoplasmosis – look in the eyes
- Single lesion more typical of lymphoma
- PML – progressive multifocal leucoencephalopathy
- Tuberculoma
- Cryptococcoma
- Pyogenic abscess
- Nocardia
- Neurocystercercosis
- CNS syphilis
90% probability of toxoplasma encephalitis if HIV +ve, CD4<100 and:
- IgG positive
- No effective prophylaxis was being taken
- Multiple ring enhancing lesions on neuroimaging
- If all 3 of the above are not present consider biopsy or other diagnostic tests
Cryptococcus:
- Raised opening pressure >20 cm on a lumbar puncture.
- Can be diagnoses with an India ink stain, culture, serology (serum CrAg) with Latex agglutination and ELISA test or lateral flow assay or a CSF cryptococcal antigen (CrAg).
- Treat with amphotericin (dosing can vary depending on formulation) and flucytosine 100mg/kg/day (usually 2 weeks) then fluconazole 400mg/day for 8 weeks then fluconazole 200mg/day until immune reconstitution. Patient may also need therapeutic LPs (in fact probably the only patients in the world that will beg you to do anther LP to ease their headache!)
- Start ART after 4 weeks of antifungals – due to the risk of IRIS.
See braininfectionuk.org for specific learning modules.
Q24. How do you diagnose children with HIV?
Answer and interpretation
All infants to HIV+ve mothers will have transplacental HIV antibodies. The mean loss is by 9-10 months. Most by 12 months and all by 18 months.
In HIV exposed infants virological testing is recommended testing can include a dried blood spot or plasma for HIV DNA or RNA PCR or p24 antigen. In HIV infected infants PCR is positive in 95% by week 4.
Because the rapid diagnostic tests look for antibodies they will only be reliable once the infant has stopped breast feeding for 6 weeks.
Western protocol for testing non-breastfed infants at HIV risk:
- During first 48 hours of birth.
- Virology 2 weeks post cessation of prophylaxis (6 weeks of age).
- Virology 2 months post cessation of prophylaxis (12 weeks of age).
- HIV antibody testing for seroconversion at 18 months of age.
Western protocol for testing breastfed infants at HIV risk:
- As above with the addition of monthly PCR testing while breast feeding.
- Then 2 and 8 weeks after weaning.
- Finally, HIV antibody testing for seroconversion at 18 months of age.
In low middle income countries HIV protocol where the above can not be achieved:
- Virology 2 weeks post cessation or prophylaxis (6 weeks of age)
- Virology at 9 months of age – last immunisation (test earlier if the child is unwell).
- HIV antibody testing for seroconversion at 18 months of age.
Infants with an initial positive virological test should be started on ART immediately and a second specimen should be sent to confirm the results. Do not delay ART while waiting for these results, the CHER trial showed mortality benefit with early treatment. Children should also be started on PCP prophylaxis.
Q25. What are the recommended treatment options for children with HIV?
Answer and interpretation
Preferred treatment options:
- Infants born to mother with HIV = AZT (twice daily) and NVP (once daily) for 6 weeks regardless of feeding method. Infants who continue to be breastfed should continue with the prophylaxis for a further 6 weeks.
- < 3 years = Lop/r (or NVP) + ABC (or AZT) + 3TC.
- 3 to 10 years = Option 1: ABC + 3TC + EFV (or NVP). Option 2: AZT (or TDF) + 3TC (or FTC) + EFV (or NVP).
- 10-19 years >35kg = TDF + 3TC (or FTC) + EFV.
Post-exposure prophylaxis regimens (<10 years of age):
- AZT + 3TC
- ABC + 3TC
- TDF + 3TC
Q26. What are the rates of mother to child transmission (MTCT) and should the child breastfeed?
Answer and interpretation
Transmission: Prior to ART minimal estimates for HIV transmission for infants who were breast-fed for 18–24 months were as follows:
- Intrauterine 5%
- Intrapartum 10%
- Breastfeeding 10% (5% before 2 mths and 5% after 2 mths of age).
- In some studies the estimates are as high as 45-50% when including all infants up to 1 year of life.
Methods of reducing transmission:
- Decrease viral load in mother (ARTs).
- Treat STIs.
- Fix anaemia and malnutrition in mother.
- Rupture of membranes <4 hours.
- Caesarean section can reduce transmission by 50% (although debated if viral load undetectable).
- Stop mixed feeding (breast milk plus other).
Breast feeding:
Infants born to HIV-infected mothers who are uninfected at delivery may be infected through breast-milk after birth especially in the first 6-8 weeks of life. A randomised, controlled trial in Kenya found a HIV transmission of 37% in breast-fed compared with 20.5% of artificially-fed infants.
However, in low middle income countries, a bottle-fed infant might have up to six times greater risk of dying than a breastfed infant in the first 2 months of life, and 3-4 fold in the next 4 months. This equates to a higher risk of dying than a non-HIV-infected infant born to an HIV-infected mother who breast feeds. Also exclusively breastfed infants have a lower risk of vertical transmission than mixed, artificial/breastfed infants. Mixed feeding (breast plus other milk) can double the transmission risk and addition of solids increases the risk by 11-fold. HIV-infected mothers should be given counselling and advised to exclusively breast feed their infants until at least 6 months and advice on appropriate complementary feeds after 6 months. ARVs should be given to all breastfed infants and breast feeding should be continued for at least 12 months and weaning encouraged only if a safe alternative can be provided.In the western setting where bottle feeding is safe, the risks of breastfeeding are too high to advocate its use.
Management of mother to child transmission (MTCT) postnatal:
• ARVs triple for mother (for example TLE – TDF/3TC/EFV).
• Infant NVP for 6 weeks if mother on ART otherwise if there is a high risk NVP + Zidovudine BD. If breastfed and high risk the length or prophylaxis can extend to 12 weeks.
• Exclusive breast feeding for 6 months (in the low middle income setting – not Western setting).
• Complementary feeds at 6 months.
• Continue breast feeding for at least 12 months (not Western setting).
• Co-trimoxazole from 4 week to 18 months of age until HIV status can be established. CHAP study showed a reduction in deaths by an absolute of 14%.
HIV infected infants:
• Exclusive BF for 6+ months
• Complementary diet from 6 months
• Co-trimoxazole 1 month of age and longterm
• Vitamin A + zinc + nutrition
• Immunisations
• ART
Immunisations, should be given as normal except:
• Two doses of measles vaccine should be given, e.g. at 6 and 9 mths.
• If available, conjugate pneumococcal (very important) and rotavirus vaccines should be given.
• Yellow fever is contra-indicated in infants with HIV-symptomatic infection.
• Complications have been described following BCG vaccination, e.g. local disease (e.g. ipsilateral axillary lymph node disease) and disseminated BCG. It is estimated that approximately 1:100 HIV-infected infants ≤1yr vaccinated at birth may develop disseminated BCG. Also in children given ART there is increased risk of BCG complications as part of IRIS. Thus if an infant is known to be HIV-infected they should not receive BCG. However, this has practical implications in low resource countries where it requires delaying BCG until the infant’s HIV status is known using PCR at 6w or later. However, due to the poor cell mediated response to BCG by HIV-infected children it may not provide much benefit anyway. In HIV-exposed, but uninfected infants, BCG immune response seems not to be impaired if BCG administration is delayed until 6-8w of age.
Additional Resources
- Online ID courses – Braininfectionsuk.org
- HIV and vaccines – BHIVA guidelines
- HIV dermatology video:
References
- Beeching N and Gill G. Lecture Notes – Tropical Medicine. 7e Wiley Blackwell 2014.
- BHIVA – The routine investigation and monitoring of adult HIV-1-positive individuals – guidelines 2016
- BHIVA/BASHH – guidelines on the use of HIV pre-exposure prophylaxis(PrEP) 2017
- BHIVA – Management of HIV infection in pregnant women 2018
- BHIVA – Management of TB/HIV co-infection in adults 2017
- Boer K, et al. Mode of delivery in HIV-infected pregnant women and prevention of mother-to-child transmission: changing practice in Western Europe. HIV Med 2010; 11:368-78.
- Braininfectionsuk.org
- Bwakura-Dangarembizi M et al and the Antiretroviral research for Watoto (ARROW) Trial Team. A randomised trial of prolonged co-trimoxazole in HIV-infected children in Africa. N Eng J Med 2014; 370:41-53.
- De Cock KM, et al. Prevention of mother-to-child transmission in resource-poor countries. JAMA 2000; 283:1175–82.
- Eddleston, Davidson, Brent, Wilkinson. Oxford Handbook of Tropical Medicine. Oxford Medical Handbooks. 4e 2014
- Evans C, et al. HIV-exposed uninfected infants in Zimbabwe: insights into health outcome in the pre-antiretroviral therapy era. Front Immunol 2016; 7:190.
- Kuhn L, et Survival and health benefits of breastfeeding versus artificial feeding in infants of HIV-infected women: developing versus developed world. Clin Perinatol 2010; 37:843-64.
- Maartens G, et al. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet 2014; 384: 258-79.
- MSF – HIV and infection
- Newell M-L, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364:1236–43.
- Nduati R, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomised clinical trial. JAMA 2000; 283:1167–74.
- Rollins NC, et al. Exclusive breastfeeding, diarrhoeal morbidity and all-cause mortality in infants of HIV-infected and HIV uninfected mothers: an intervention cohort study in KwaZulu Natal, South Africa. PLoS One 2013; 8:e81307.
- UK Guideline for the use of HIV Post-Exposure Prophylaxis Following Sexual Exposure (PEPSE) 2015.
- WHO – The use of antiretroviral drugs for treating and preventing HIV infection– guidelines 2016 update.
- WHO – HIV prevention, diagnosis, treatment and care for key populations – guidelines 2016
- WHO – HIV testing services – guidelines July 2015
CLINICAL CASES
Tropical Travel Trouble
Peer Reviewer
• Dr McBride ID physician, Wisconsin
Dr Neil Long BMBS FACEM FRCEM FRCPC. Emergency Physician at Kelowna hospital, British Columbia. Loves the misery of alpine climbing and working in austere environments (namely tertiary trauma centres). Supporter of FOAMed, lifelong education and trying to find that elusive peak performance.