Total TB Extravaganza
aka Tropical Travel Trouble 008
TB affects 1/3rd of the population and one patient dies every 20 seconds from TB. Without treatment 50% of pulmonary TB patients will be dead in 5 years. In low to middle income countries both TB and HIV can be ubiquitous, poor compliance can lead to drug resistance and malnourished infants are highly susceptible.
TB can be very complex and this post will hopefully give you the backbone to TB management, its diagnosis and a starter guide to treatment. All TB patients should be discussed with an expert.
Q1. What is Tuberculosis (TB)?
Answer and interpretation
- A disease caused by the bacterium Mycobacterium tuberculosis.
- It is an acid-fast fungus-like bacterium.
- Slow generation time – approx 17hrs which is why it can take weeks to culture.
Q2. How do you get TB and what is active vs latent infection?
Answer and interpretation
Inhalation – hence the N-95 mask and negative pressure room.You can only be infected via an airborne contact with TB droplet nuclei.
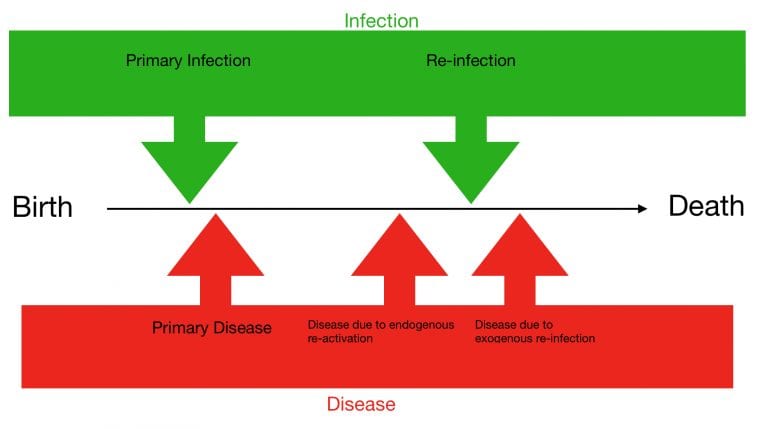
Once the mycobacterium are inhaled you develop a primary infection. This will cause a primary disease (primary tuberculosis – active disease), often presenting as a flu like illness (exception being miliary disease whereby the patient will likely be septic). In most cases the immune system will encase the mycobacterium (granuloma) as opposed to full infection in an immunocompromised host. The tissue in the centre of the granuloma will die resulting caseous necrosis (Gohn focus).
Two options are possible from a Gohn focus, either the bacteria has been killed off or some lie dormant (latent TB). From here the TB may remain dormant or reactivate (typically when a patient’s immune system is compromised). This is called re-activation and also represents active infection. The baseline risk of re-activation is 5-10% in a lifetime but approximately 10% per year in an HIV positive patient (strongly associated with the CD4 count). Patients with latent TB can not spread the disease unless it re-activates. In other words, Latent TB is non-infectious. Only active pulmonary TB can spread the mycobacterium.
The diagram below illustrates the possible outcomes of TB. You can have a primary infection and develop primary disease or it can re-activate later (or be completely cleared). It is also possible to be re-exposed and develop a re-infection. Just because you have had TB once does not make you immune to being infected later in life (a problem faced in high endemic areas of TB raising the question of whether you should treat or not treat latent TB).
Q3. How do you diagnose active TB?
Answer and interpretation
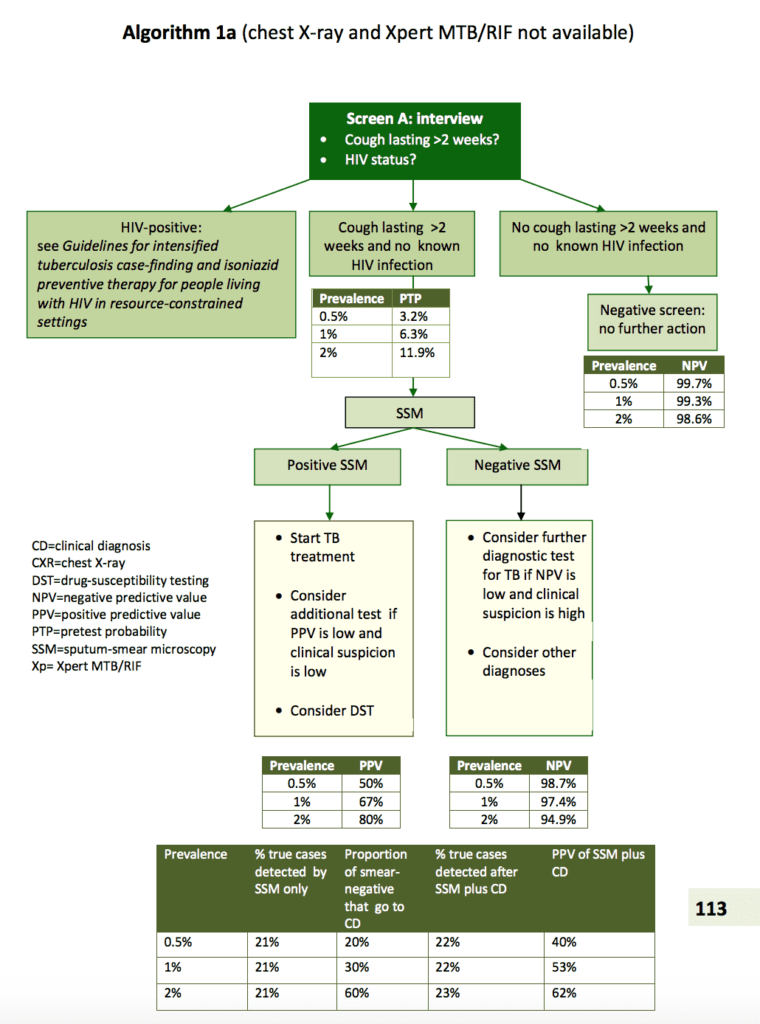
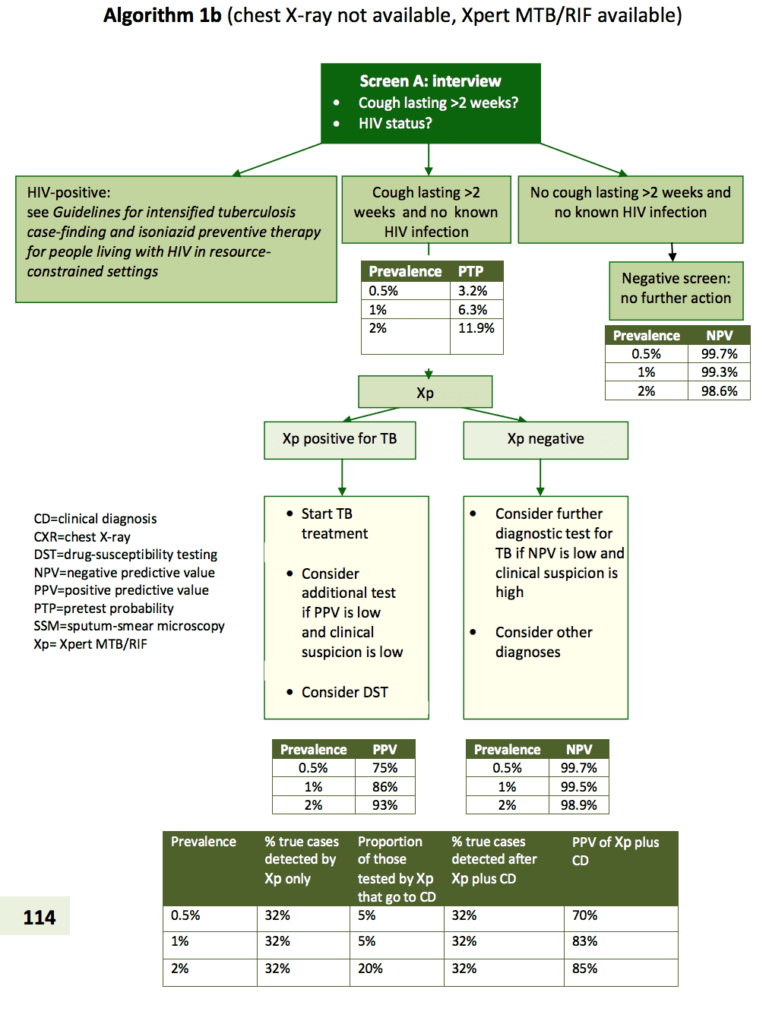
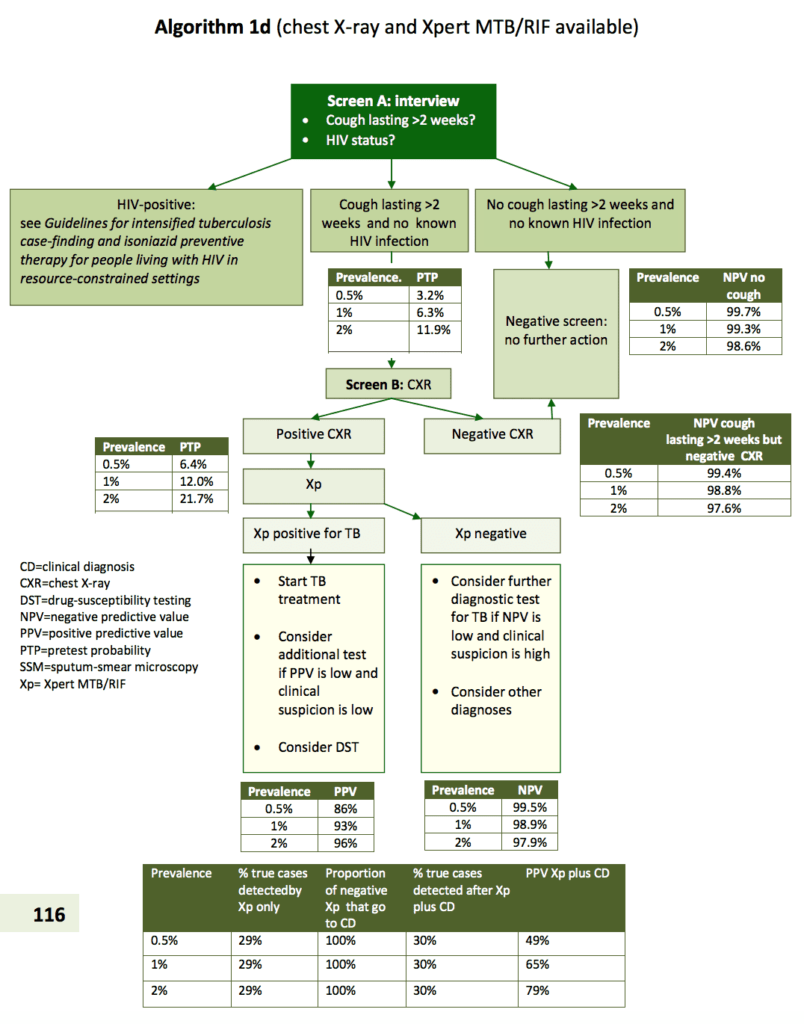
In summary, culture is the gold standard but takes time. A good clinical screen, sputum smears and chest X-ray are done in most resource poor settings. The new addition to most countries has been the GeneXpert which is a mini PCR machine that can detect TB DNA (see below for more details).
Clinical Diagnosis:
- Cough – usually productive
- Fever
- Weight loss
- Night sweats
- If negative for all the above and a negative CXR = high negative predictive value for active pulmonary TB (can not rule out extra pulmonary TB).
CXR
- The initial focus of infection can be located anywhere within the lung and has non-specific appearances ranging from too small to be detectable, to patchy areas of consolidation or lobar consolidation.
- Radiographic evidence of parenchymal infection is seen in 70% of children and 90% of adults.
- Cavitation is uncommon in primary TB, seen only in 10-30% of cases.
- Ipsilateral hilar and contiguous mediastinal (paratracheal) lymphadenopathy, usually right-sided. 90% of paediatric primary TB, but only 10-30% of adults.
- Pleural effusions, 30-40% of adults, 5-10% of paediatrics.
- As the host mounts an appropriate immune response both the pulmonary and nodal disease resolves. Calcification of nodes is seen in 35% of cases.
- Non-radiologists, sensitivity 60-70%, specificity 50%. Radiologists are 10% better.
- For images and further details on findings as the disease progresses please follow this radiopedia
Sputum smears – looking for acid fast bacilli, stained with Ziehl-Neelson.
- Sensitivity 60-70% with >90% specificity (fluorescent microscopy gains an extra 9% in sensitivity)
- Classically 3 sputum samples are requested every morning for 3 days. Trials have shown that 2 spot samples on day one and a morning sample on day 2 (or any combination of spots and one morning sample) is just as effective as 3 morning samples and reduces patient drop out. This is obviously country, patient and setting specific.
- No increased yield from performing more invasive strategies above asking for a repeat sputum. Induced sputum, gastric wash (except infants who can not expectorate) or BAL did not improve the yield.
Sputum culture gives an addition 20-30% yield and can be used to establish drug sensitivities and resistance but takes time to grow (2-3 weeks in liquid medium vs 6-8 weeks on solid medium)
PCR endorsed by WHO:
- GeneXpert – 83-93% sensitive. It is an automated mini nucleic acid amplification test. Any fluids can be put into the cartridge but typically sputum, CSF and urine is used.
- Xpert MTB/RIF can determine if there is an rpoB mutation and hence rifampicin (rifampin in USA) resistance as well as TB infection. Its presence is part of many algorithms across the world.
- Result in 2 hours, sensitivity similar to culture.
- Cost $10 per test.
- Xpert Ultra 70-95% sensitive for TB meningitis (Xpert and Culture 43%).
- Line probe assay – Used in well resourced laboratories to detect multiple drug resistance by PCR. For example, assay MTBDRplus is a molecular probe capable of detecting rifampicin- and isoniazid-resistance mutation (rpoB gene for rifampicin, katG and inhA genes for isoniazid resistance).
- Tissue biopsy (do not place specimens in formalin) or needle aspiration of pus (i.e. in a cold abscess).
- Urine LAM antigen – Urine-based detection of mycobacterial cell wall glycolipid lipoarabinomannan more commonly used in those with HIV and has a better sensitivity with CD4 counts below 200 (sensitivity 71%).
Algorithms for TB diagnosis as per WHO:
Q4. How do you diagnose latent TB?
Answer and interpretation
Testing is required to prove you have been exposed to TB in the past. There are two options:
TST (tuberculin skin test): Tuberculin purified protein derivative (PPD) is derived from human strains of M. tuberculosis, and consists of several antigenic components.
- All available tuberculins are subject to significant cross-reactivity with other species of mycobacteria, including BCG-bovis, and environmental mycobacteria, M. avium complex (MAC). This results in a significant reduction of both sensitivity and specificity.
- A positive response on skin testing is therefore a measure of previous exposure/infection potentially to several mycobacterial species.
Disadvantages of TST:
- Poor specificity: high false positive results from previous BCG vaccination, a single BCG vaccination at birth (usually wanes over the next decade, however, BCGs given after age 2, and repeat BCGs can lead to prolonged (false) positive TST), exposure to non-tuberculous mycobacteria (NTM).
- Poor sensitivity: immune dysfunction and other factors cause false negative TST results. Examples include: HIV, and other immunosuppressive diseases or therapy, inadequate nutrition, malignancy, active TB, especially severe/miliary TB, concurrent viral infection, children and the elderly.
Interpretation of TST in the absence of BCG vaccination: Three cut-off diameters have been recommended for defining a positive reaction: ≥ 5 mm, ≥ 10 mm and ≥ 15 mm dependent on patient risk groups:
- For persons at highest risk for developing TB disease, if they become infected with M. tuberculosis, a cut-off of ≥ 5 mm is recommended (for example, HIV-positive or otherwise immunosuppressed persons, all close contacts of active smear-positive cases).
- A reaction of ≥ 10 mm should be considered positive for those with an increased risk of recent infection (for example, recent immigrants and IV drug users).
- ≥ 15 mm for all others.
Interferon (IFN) gamma release assays (IGRAs): T-cells of individuals previously infected with M. tuberculosis will produce IFN‑gamma when they encounter TB-specific mycobacterial antigens. The antigens used in IGRAs (ESAT-6, CFP-10 with or without TB 7.7) are encoded by M. tuberculosis genes that are not shared with M. bovis-BCG or most NTM, making the test more specific.
- Advantages over TST: better specificity in the setting of BCG vaccination and NTM exposure, similar sensitivity to TST in most cases, although IGRAs may be more sensitive in the setting of immune suppression, does not require return visit, automated interpretation of QFN-GIT eliminates problems with inter-user reliability, no booster phenomenon.
- Disadvantages: IGRAs are more expensive than TST, IGRAs may not perform as well in children as in adults, and currently the CDC and WHO suggest that in children < 5 years TST is preferred, although QFN-GIT is an acceptable alternative. The WHO also state TST is preferred in low middle income countries.
Q5. What is the treatment for drug susceptible TB?
Answer and interpretation
The backbone of most treatment regimens is:
- Rifampicin (R) (best drug for treating TB)
- Isoniazid (H)
- Pyrazinamide (Z)
- Ethambutol (E)
RHZE in Australia/NZ/UK, RIPE in North America (Rifampin, Isoniazid, Pyrazidime and ethambutol).
- Intensive phase 2 months RHZE / Continuation phase 4 months RH. Most patients can be treated as an outpatient with very few requiring an admission.
- Shortened courses involving fluoroquinolone are being studied but currently not recommended by the WHO.
- Smear or culture negative in the last months of treatment and one before cessation of treatment is used to confirm “cure”. Some practitioners advocate a negative smear before changing from the intensive phase to continuation phase.
- RHZE has an 85% cure rate. Only a small percentage of failure is due to primary drug resistance (acquiring a drug resistance strain). The majority of failed regimens are due to poor medication compliance or supply of medication. It is imperative that patients are educated to take their medication as prescribed. Failure to cure the initial TB infection leads to months or years of ongoing treatment.
- See TB drug monographs for full prescribing details for all TB drugs.
Q6. Who is not suitable for 6 months of treatment?
Answer and interpretation
- Disseminated TB (including miliary): 9-12 months.
- CNS TB: 12 months.
- Skeletal TB: at least 9-12 months.
- Drug resistant TB (20 months).
Q7. What are the potential side effects of RHZE treatment?
Answer and interpretation
Rifampicin (R) – Hepatitis, GI upset, skin rashes, thrombocytopenia, renal failure, haemolytic anaemia, orange discolouration of bodily fluids, frequent drug interactions (see HIV section) and drug induced SLE.
Isoniazid (H) – Hepatitis, seizures, peripheral neuropathy (preventable with pyridoxine – vitamin B6) and hypersensitivity reactions.
Pyrazinamide (Z) – Hepatitis, arthralgia, flushing, gout, lability of blood glucose in diabetics. Top tip: while RHZ are associated with hepatitis, the most common culprit is pyrazinamide.
Ethambutol (E) – optic neuritis (avoid in children <7 years – discuss with a specialist for advice).
Q8. How do you treat latent TB?
Answer and interpretation
Isoniazid preventive therapy for 6 months after exclusion of active TB.
Alternative regimens:
- Rifampicin plus isoniazid daily for 3 months should be offered as an alternative to children <15 years.
- Rifapentine and isoniazid weekly for 3 months in both adults and children.In fact many countries are turning to this as the most convenient protocol.
- Rifampicin alone in countries with low TB incidence.
If HIV positive should have at least 36 months of IPT (according to WHO, and probably relates to high endemic TB settings – expect different protocols in the developed world).
Q9. How do you treat drug resistant TB?
Answer and interpretation
Golden rule: due to its rarity in some countries, including the UK, each patient should be discussed with a specialist and with a MDRTB centre or forum.
Do not add one drug to a failing regimen. Always add two. This is a general rule but will depend on the information you have to hand.
Definitions used in the guidelines:
Monoresistance: resistance to one first-line drug, usually isoniazid.
– Multi drug resistant = resistant to rifampicin and isoniazid
– Extensive drug-resistance (XDR): MDR plus
Resistance to any fluoroquinolone and…
Resistance to any second line injectable (Kanamycin, capreomycin or amikacin)
– Total drug resistance (TDR): Not clearly defined and not recognised by WHO due to drug-susceptibility testing may not be accurate in some settings, newer drugs are being developed and newer evidence on previously less-used drugs.
– Genotypic resistance tests (rapid molecular test) / Phenotypic resistance test (culture)
– Cleared once 3 sputum smears are negative ideally a week apart + completed course of antibiotics.
• Treatment options:
–Isoniazid monoresistance = 2RZE + levofloxacin for 6 months. Need to make sure there is no rifampicin or levofloxacin resistance. Do not give if prolonged QTc, pregnant or breast feeding.
– Rifampicin monoresistance = Treat as MDRTB
– MDRTB (see table below):
• Intensive phase 8 months (must be smear negative before moving on).
• Needs at least 5 effective TB medications including pyrazinamide unless resistant and an injectable.
• Chose one from group A, one from group B and at least 2 from group C. If this is not possible, an agent from group D2 and other agents from group D3 may be added to bring the total to 5 agents.
• The regimen can also be strengthened by giving high-dose isoniazid and/or ethambutol.
• Continuation phase a further 12 months, stop injectable and continue on at least 3 other drugs. However, if they have never been on second line agents and are susceptible to these agents selected plus fluroquinolones, a shorter MDR-TB regimen of 9-12 months may be used instead (low certainty of evidence).
Q10a. TB can occur at other sites (it’s always on a medical student differential and essentially if you think of an organ … it can have TB), can you match the photos below to some of the TB presentations?
Answer and interpretation
12% of TB cases are extra pulmonary. More common in low CD4 counts, almost half of TB in HIV +ve patients in sub-Saharan Africa is EPTB and most are not diagnosed in their lifetime.
- Image 1 – Pleural – a subtle lesion is in the right lower zone coming from the chest wall with acute angles. This requires a pleural biopsy as a pleural fluid smear is 2% sensitive, culture 40% sensitive and 100% specific (gold standard). Raised adenosine deaminase 90% sensitive and specific. GeneXpert 54% sensitive and 99% specific. Treat for a total of 12 months, 2 months intense and 10 months continuation
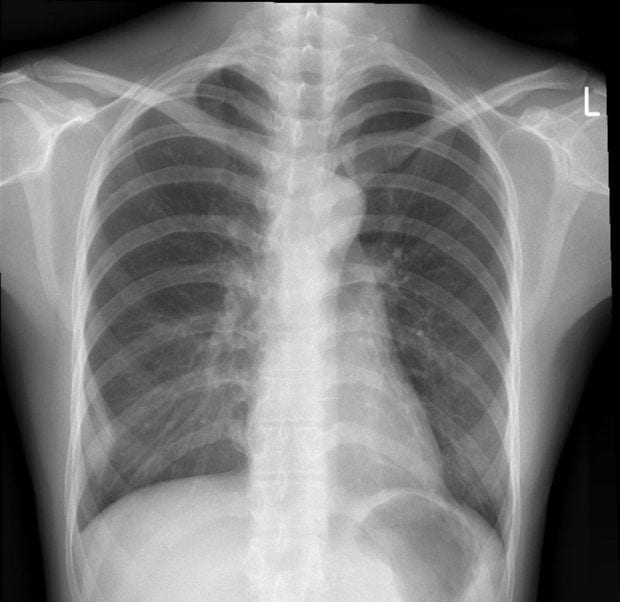
- Image 2 – Lymph node (scrofula), can cause a sinus or fistula formation.
- Image 3 – TB pericarditis – as seen by calcification of the pericardial sac, an initial adjuvant corticosteroid therapy may be used to prevent constrictive pericarditis (60mg/day and gradually withdraw after 2-3 weeks of treatment).
Q10b. Can you match the following photos to some more TB presentations?
Image 4 Image 5a Image 5b Image 6
Answer and interpretation
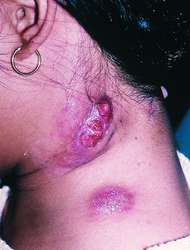
- Image 4 – CNS – TB meningitis (in detail below), arachnoiditis, tuberculoma (seen in the image).
- Tuberculoma — Tuberculomas are conglomerate granulomatous foci within the brain parenchyma. Clinically silent single or multiple nodular enhancing lesions are commonly seen in the setting of meningitis; occasionally, they are seen in patients with miliary tuberculosis and no meningitis. These lesions generally disappear on therapy but may heal with calcification. Treatment is as per TBM but does require the continuation phase to extend to 18 months instead of 10.
- Image 5a+b – Bone and spine (Gibbus formation or Pott’s disease) – patients may present with neurological deficit or can been seen clinically with severe kyphosis, expert opinion states 9-12 months of treatment. Drainage of any abscesses and resection of diseased bone, spinal fusion or bone grafting may reduce deformity and reduce the chance of permanent neurological deficit.
- Image 6 – Renal – the image is of TB epididymitis but leads into a number of renal conditions that TB can cause: papillary necrosis, stricture formation, calyceal dilatation, ureteric obstruction and sterile pyuria. And for genitourinal: scrotal, epididymal mass, scrotal sinus, chronic prostatitis, epididymitis. Diagnosis with early morning urine for culture and GeneXpert. (see Dr McBrides recent publication – wrong fame of mind – for a recent case)
Q10c. Can you match the following photos to some more TB presentations?
Answer and interpretation
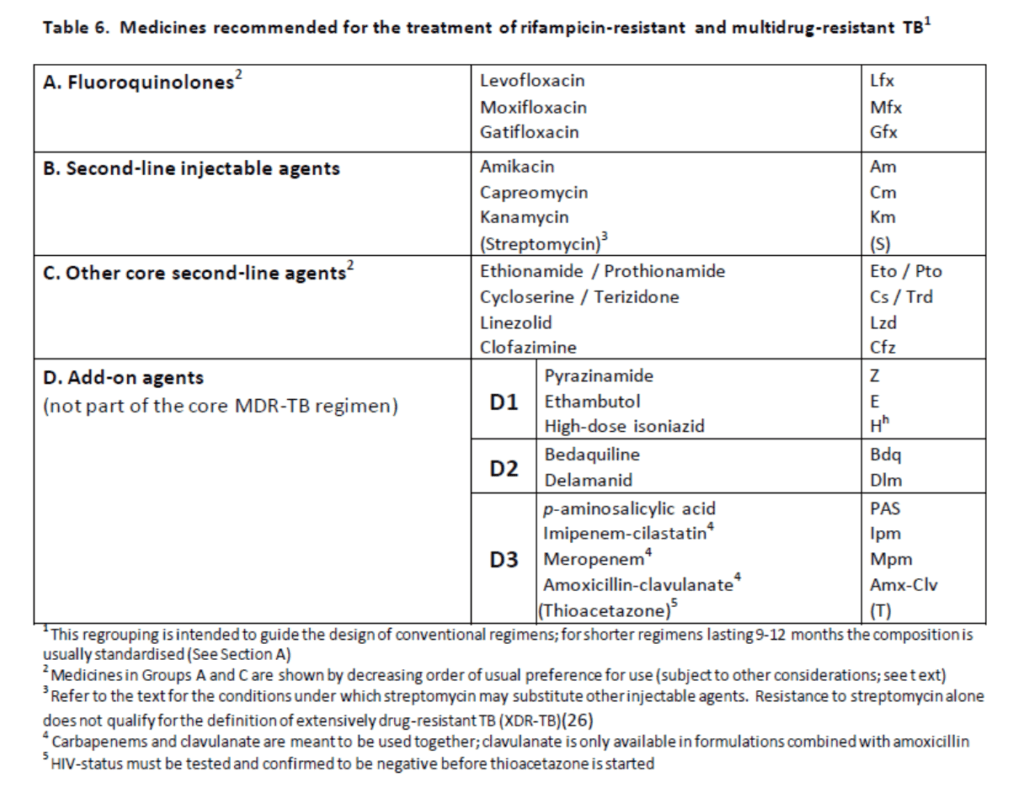
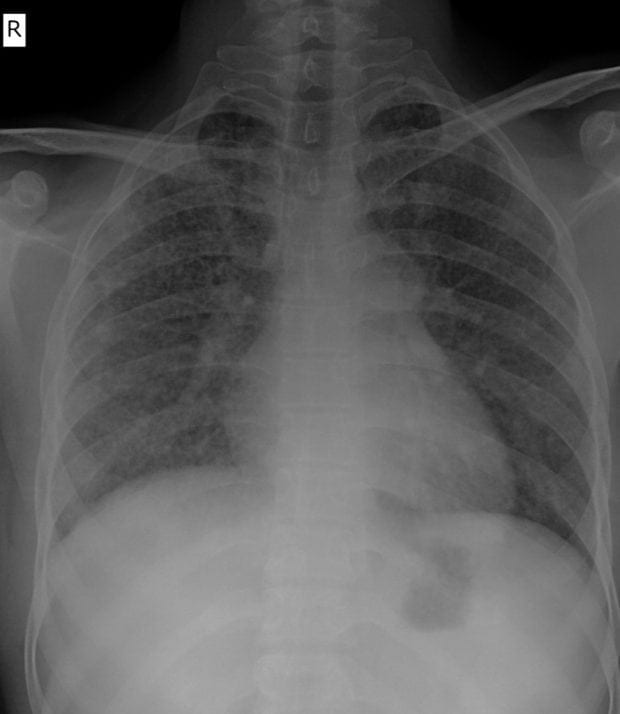
- Image 7 – Disseminated and miliary TB is shown in the CXR, high mortality up to 30% and if the patient has drug resistant TB it is likely to be fatal.
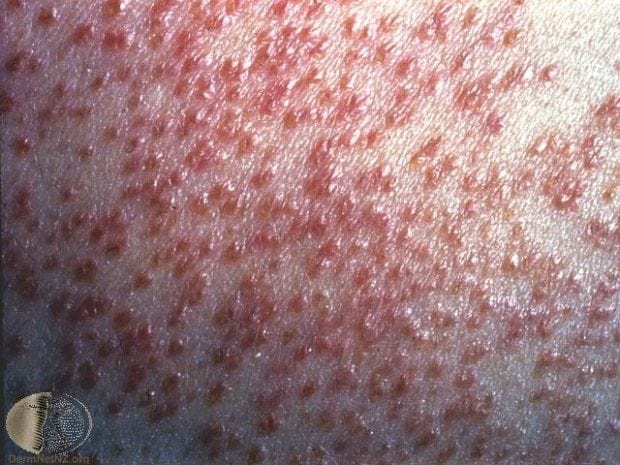
- Image 8 – Skin, the image shown is of miliary TB but TB skin disease can manifest in a variety of ways, see dernetnz for more images.
- Image 9 – Gastrointestinal – commonly TB is found in the terminal ilium. Tuberculous enteritis, ulceration (seen in the image), pseudo tumour, inflammatory mass formation and stricture.
Q11.TB Meningitis (TBM) is the most severe form of TB, mortality 20-40% with disability common in survivors. Drug resistant TBM with HIV co-infection has a near 100% mortality. What are the symptoms, signs, investigation and treatment options for TBM?
Answer and interpretation
Symptoms are subacute: 1-3 weeks, headache, vomiting, conscious level, confusion, constitutional symptoms, seizures
Signs: choroidal tubercles on ophthalmoscopy; cranial nerve palsies develop (5th and 3rd most common in 50% of patients); limb weakness either hemiplegia or paraplegia in 10%; TB elsewhere (1/3rd of patients will have miliary TB); and declining GCS.
MRC Clinical Grading:
- = Alert and orientation without focal neurological deficit, 9.4% mortality.
- = GCS 10-14 with or without focal neurological deficit or GCS 15 with focal neurological deficit, 18.6% mortality.
- = GCS <10 with or without focal neurological deficit, 44.5% mortality.
Investigations:
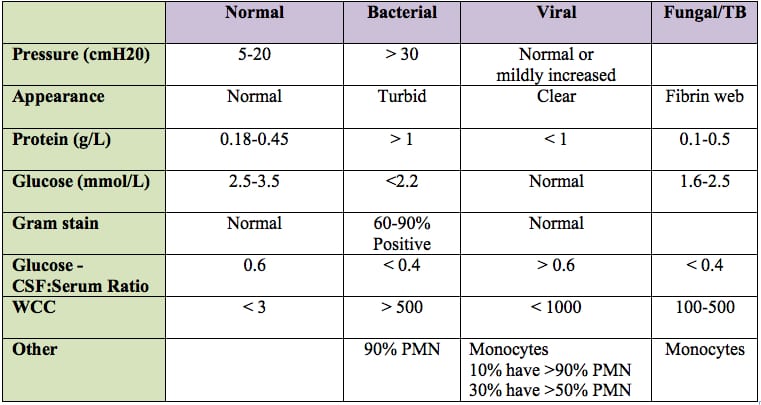
- CSF findings: Lymphocyte pleocytosis, raised protein 0.8 to 2.0 g/dL, raised opening pressure, CSF: Plasma glucose levels <0.5 in 90% of individuals.
- Unfortunately with HIV the CSF results can resemble a more classic bacterial meningitis picture or have no leukocytes present.
- AFBs are rarely seen in routine samples.
- Need large volumes of CSF to improve sensitivity (10ml – 15ml) and some centres recommend doing daily LPs until four are achieved to increase your diagnostic yield from 37% to 87% (requires an expert to examine the smear for at least 30 minutes).
- See table below for similar figures (textbook answers vary).
- CXR to show signs of TB infection or miliary disease (normal in 50% of patients with TBM).
- CTB and MRI improve the diagnosis, looking for basilar arachnoiditis, cerebral oedema, infarction, tuberculoma and hydrocephalus. The CT can be normal in 30% of patients with stage 1 disease. Spinal cord disease is common in people with TBM and often goes undetected. One imaging study of children with TBM and hydrocephalus found 76% had spinal disease.
- Adenosine deaminase assay is elevated in TB meningitis but also in bacterial infection and there is no clear threshold to distinguish between the two.
- Nucleic acid amplification test – GeneXpert MTB/RIF or GeneXpert Ultra (45% and 95% sensitive respectively).
Treatment:
- WHO recommends 2 months RHZE and 10 months RH, same dosing as per pulmonary TB despite rifampicin only reaching 10-20% concentration in CSF plasma and ethambutol does not cross the blood brain barrier, even when it’s inflamed. Paediatric dosing is higher.
- Plus steroids (dexamethasone or prednisolone) over 6-8 weeks. RCTs showed lower rates of mortality, death or severe disability.
- The treatment regimen for MDR TBM is unknown and currently the recommendations are to treat as per MDR in pulmonary TB but select drugs which can penetrate the blood brain barrier in high concentrations.
Evolving research (watch this space):
- Higher dose rifampicin regimens are having positive clinical outcomes. Adults increasing 450mg to 600mg of rifampicin and 30mg/kg orally or 15mg/kg intravenous in children.
- The choice of a 4th drug has been controversial but overall there appears to be no difference in outcome if the bacteria is susceptible to all first line agents. However, in isoniazid-resistantance, clinical trial data indicate that a high dose (minimum 15mg/kg) of rifampicin and the addition or levofloxacin as a fifth agent improves survival. Observational data also indicates that the negative effect of isoniazid resistance on survival could be reduced by prescribing pyrazinamide throughout treatment (9-12 months).
- There are 2 studies showing that the addition of aspirin is beneficial in preventing strokes but these are small studies.
- See braininfectionuk.org for a learning module on TB meningitis.
Q12. What are the differences in children with TB (diagnosis, management, children born to mothers with TB)?
Answer and interpretation
The diagnosis and management of TB in children (<10yrs) is different in a number of aspects to that in older children and adults. This is particularly so for diagnosis of pulmonary TB as young children (<5yr) do not expectorate sputum and their disease is paucibacillary.
Its also important to think of children as ‘canaries in the coal mine’, they are rarely infectious as they infrequently have cavitary disease and often lack the expiratory force required to expel the droplet nuclei. Therefore, when you see a child with TB you need to look at the other family members to see who has it.
Diagnosis:
- Based on a history of a persistent cough (>2 weeks), persistent fever without clear aetiology, contact history (within 24 months), plus a positive tuberculin skin test and a chest radiograph compatible with TB.
- In low- and middle-income countries (LMIC) most children who have received BCG at birth will have a negative TST by 2-3 years. So, in general, previous neonatal BCG can be ignored when interpreting the TST after this age.
- Gastric aspirate < 12months of age is 70% sensitive if cultured as apposed to induced sputum at 30% or nasopharyngeal aspirate 25%.
- GeneXpert sensitivities are 66% sputum, 66% gastric aspirate and 45% stool sample. Thus as a stand alone test the GeneXpert should not be relied upon and culture or urine LAM should be also considered (resource depending).
- The TST may be negative in young infants and immunosuppression associated with malnutrition, HIV infection and current viral infections, e.g. measles. If negative it should be repeated after the child has responded to chemotherapy when it may become reactive and help to support the diagnosis.
- Interferon -γ release assays (IGRA) are less sensitive in children <5 years of age than adults, thus the WHO’s recommendation for TST.
- Urine lipoarabinomannan (LAM) antigen assay is especially useful for diagnosis in HIV-infected children with low CD4 counts (<200).
- HIV testing should always be offered in conjunction with TB screening.
Management:
- Asymptomatic Primary Infection (positive TST, normal chest radiograph). Same as per adults, isoniazid for 6 months.
- Symptomatic Disease (except TBM and osteoarticular TB). Same as per adults, Rifampicin (R), Isoniazid (H), Pyrazinamide (Z) and Ethambutol (E) for 2 months and then isoniazid and rifampicin for 4 months. For non-HIV-infected children with low risk of INH resistance and non-extensive disease HRZ can be given and ethambutol omitted. Due to the possibility of retrobulbar neuritis caution is required when giving ethambutol to young children who may not be able to report visual symptoms; the dose should not be above 20mg/kg (seek expert help for dosing regimen).
- Extra pulmonary TB (Tuberculous meningitis): are treated as per adult regimens. For TB meningitis and pericarditis corticosteroids are given to suppress the inappropriate inflammatory response (IRIS). Prednisolone 2-4 mg/kg/day or dexamethaxone 0.6 mg/kg/day is given for approximately 4 weeks and gradually tailed off over 2-4 weeks. The high dose of prednisolone is recommended because rifampicin enzyme induction lowers the level of prednisolone by up to 50% . A third of children with miliary disease may have CNS involvement so a lumber puncture should be undertaken to exclude TBM.
- Infant born to a mother with tuberculosis: At birth, if the mother has active disease or is receiving treatment the infant is given isoniazid 10 mg/kg for 3 months. Once the mother and infant are on appropriate treatment the infant may be breast-fed unless the mother has MDR-TB. At 3m a TST is performed. If the infant is well and the test is negative, a BCG is given and he/she is discharged (chest radiograph is optional). If the TST is positive or there are symptoms a chest radiograph (and gastric aspirate for smear & culture if available) is done and if TB is suspected full chemotherapy is given. If the mother has a history of TB (i.e. no active disease) at delivery and the infant is well just a BCG is given. If the mother has HIV then the infant is investigated and followed up as appropriate.
Drug dosage (mg/kg):
- Isoniazid* 10 mg (7-15) (max 300).
- Rifampicin 15 mg (10-20) (max 600).
- Pyrazinamide 35 mg (30-40).
- Ethambutol 20 mg (15-25).
- Ethionamide 15-20 mg.
* The lower range of INH (7 mg) is to suit the fixed combination tablet. For INH prophylaxis the dose should be 10-15 mg/kg.
Q13. TB and HIV are often co-existing in some areas, how do you screen for TB in these individuals and when would you start antiviral therapy (ART)? Also what is IRIS?
Answer and interpretation
Adults and adolescents living with HIV should be screened for TB. If they do not report any current cough, fever, weight loss or night sweats they are unlikely to have active TB and should be offered preventative therapy (isoniazid for 6 months). CXR may be offered as an additional step to check for active disease (same protocol for HIV negative patients).
ART should be started in all TB patients living with HIV regardless of their CD4 count, it’s the timing which is important.
TB treatment should be initiated first, followed by ART as soon as possible in the first 8 weeks of treatment. Profound immunosuppression with CD4 counts <50 cells/mm3 should receive ART within the first 2 weeks of initiating TB treatment. 8 trials had high quality of evidence to show starting ART before 8 weeks improved mortality. 4 of the trials also showed the benefit was higher when started within 2 weeks. No comment from WHO in regard to TBM as a subgroup.
TBM-associated immune reconstitution inflammatory syndrome (TBM-IRIS), which comprises exaggerated inflammation in response to M.Tuberculosis. IRIS describes a collection of inflammatory disorders associated with paradoxical worsening of pre-existing infectious processes following the initiation of antiretroviral therapy in HIV +ve individuals. The immune system wakes up with ART and goes into overdrive. If your TB is a cold abscess on your back and you get IRIS, it doesn’t really matter if this swells or associated lymph nodes swell, but the same situation intracranially can potentially be life threatening – remember the Munro Kelly doctrine. From one study it has been recommended that ART-naïve HIV-infected patients with CNS tuberculosis, initiation of ART should be delayed for the first eight weeks of antituberculous therapy, regardless of CD4 count. There was no difference in survival but reduced Grade 4 adverse events (co-morbidities resulting in immobility).
TB regimens cause drug interaction with ARTs for example, rifamycins induce hepatic CYP3A4 enzymes that can accelerate metabolism of protease inhibitors and some nonnucleoside reverse transcriptase inhibitors (NNRTIs). Despite the complexity of managing drug interactions, rifamycins are integral to the success of TB therapy and should not be substituted with other antituberculosis medications based on concerns regarding drug interactions alone.
See HIV drug interactions to simplify a regimen (and speak to a specialist)
Q14. How much protection does a BCG vaccination provide against the development of pulmonary TB in developing countries?
Answer and interpretation
<40%.
There is a wide variation in the reported protective efficacy of BCG and an apparent inverse correlation between efficacy and proximity to the equator (more effective in latitudes >40 degrees). Factors that might account for these findings include differences between BCG strains, age at vaccination, nutritional (for example, vitamin D) factors, and differences in exposure to environmental non-tuberculous mycobacteria.
It protects against disseminated TB and TB meningitis in children (73% effective when given in the neonatal period)
Do not give if the infant is HIV positive.
The duration of protection following BCG vaccination is uncertain. Protection following infant vaccination is believed to wane after 10 to 20 years. It is generally believed the vaccination does not prevent infection with TB, or reactivation of LTBI, so BCG vaccination does not have a significant role in preventing transmission. BCG vaccination also has no role in the treatment of active TB but does provide some protection against leprosy caused by Mycobacterium leprae.
References
- Amogne W, Aderaye G, Habtewold A, Yimer G, Makonnen E, Worku A, et al. Efficacy and safety of antiretroviral therapy initiated one week after tuberculosis therapy in patients with CD4 counts <200 cells/μl: TB-HAART Study, a randomized clinical trial. PLoS One. 2015;10(5):e0122587.
- Beeching N and Gill G. Lecture Notes – Tropical Medicine. 7e Wiley Blackwell 2014.
- Bell DJ et al. Simple measures are as effective as invasive techniques in the diagnosis of pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2012;13(1):99-104
- Chotmongkol V, Jitpimolmard S, Thavornpitak Y. Corticosteroid in tuberculous meningitis. J Med Assoc Thai. 1996;79(2):83-90. [PMID 8868018]
- Coulter JBS. Perinatal tuberculosis. Ann Trop Paediatr 2011; 31:11-13. [PMID 21262105]
- Coulter JBS. Diagnosis of pulmonary tuberculosis in young children. Ann Trop Paediatr 2008;28:3-12. [PMID 18318944]
- Detjen AK, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med 2015: 3:451-61. [PMID 25812968]
- Donald PR, van Toorn R. Use of corticosteroids in tuberculous meningitis. Lancet 2016; 387:2585-87. [PMID 27353808]
- Donald PR, et al. Intensive short course chemotherapy in the management of tuberculous meningitis. Int J Tuberc Lung Dis 1998; 2:704-11. [PMID 9755923]
- Eddleston, Davidson, Brent, Wilkinson. Oxford Handbook of Tropical Medicine. Oxford Medical Handbooks. 4e 2014
- Graham SM, et al. Evaluation of tuberculosis diagnosis in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis 2012; 205:S199-208. [PMC3334506]
- WHO. Guidance for National Tuberculosis programmes on Management of Tuberculosis in Children. 2nd ed. WHO, 2014.
- Havlir D, Kendall M, Ive P, Kumwenda J, Swindells S, Qasba S, et al. for the AIDS Clinical Trials Group Study A5221. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–91. [PMC3327101]
- Kumarvelu S, Prasad K, Khosla A, Behari M, Ahuja GK. Randomized controlled trial of dexamethasone in tuberculous meningitis. Tuber Lung Dis. 1994;75(3):203-7. [PMID 7919313]
- Malhotra HS, Garg RK, Singh MK, Agarwal A, Verma R. Corticosteroids (dexamethasone versus intravenous methylprednisolone) in patients with tuberculous meningitis. Ann Trop Med Parasitol. 2009;103(7):625-34. [PMID 19825284]
- Matthews PC. Tropical Medicine Notebook. Oxford University Press, 2017
- Manosuthi W, Mankatitham W, Lueangniyomkul A, Thongyen S, Likanonsakul S, Suwanvattana P, et al. for the TIME Study Team. Time to initiate antiretroviral therapy between 4 weeks and 12 weeks of tuberculosis treatment in HIV-infected patients: results from the TIME Study. J Acquir Immune Defic Syndr. 2012;60:377–83. [PMID 22592586]
- Mfinanga S, Kirenga B, Chanda D, Mutayoba B, Mthiyane T, Yimer G et al. Early versus delayed initiation of highly active antiretroviral therapy for HIV-positive adults with newly diagnosed pulmonary tuberculosis (TB-HAART): a prospective, international, randomised, placebo-controlled trial. Lancet Infect Dis. 2014;14:563–71. [PMID 24810491]
- Mukherjee A, et al. Ambulatory gastric lavages provide better yields of Mycobacterium tuberculosis than induced sputum in children with intrathoracic tuberculosis. Pediatr Infect Dis J 2013; 32:1313-7. [PMID 23958816]
- Naidoo K, Yende-Zuma N, Padayatachi N, Naidoo K, Jithoo N, Nair G Bamber S, et al. Immune reconstitution inflammatory syndrome following antiretroviral therapy initiation in tuberculosis patients: findings from the SAPiT Trial. Ann Intern Med. 2012;157(5):313–24. [PMC3534856]
- NICE – TB guidelines. Jan 2016.
- Rothe C. Clinical Cases in Tropical Medicine. Elsevier 2015.
- Roy A, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ 2014; 349:g4643. [PMID 25097193]
- Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997;99(2):226-31. [PMID 9024451]
- Shao H, Crump J, Ramadhani H, Uiso L, Ole-Nguyaine S, Moon A, et al. Early versus delayed fixed dose combination abacavir/lamivudine/zidovudine in patients with HIV and tuberculosis in Tanzania. AIDS Res Hum Retroviruses. 2009;25(12):1277–85. [PMC2858925]
- Sinha S, Shekhar R, Singh G, Shah N, Ahmad H, Kumar N, et al. Early versus delayed initiation of antiretroviral therapy for Indian HIV-infected individuals with tuberculosis on antituberculosis treatment. BMC Infect Dis. 2012;12:168. [PMC3457866]
- Thwaites G, et al . British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect 2009; 59:167-87. [PMID 19643501]
- Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741-51. [PMID 15496623]
- Uptodate – Central Nervous System Tuberculosis. Accessed 2018.
- Uptodate – Diagnosis of pulmonary tuberculosis in adults. Accessed 2018.
- Wait CJ et al. The effect of a tuberculosis chest X-ray image reference set on non-expert reader performance. Euro Radio 2013;23(9):2459-2468
- WHO – Guidelines for treatment of drug-susceptible tuberculosis and patient care. 2017 UPDATE
- WHO – Guidance for national tuberculosis programmes on the management of tuberculosis in children. Second Edition 2014.
- WHO – Latent tuberculosis infection. 2018
- WHO – Systematic screening for active tuberculosis. 2013
- WHO – Treatment guidelines for drug-resistant tuberculosis 2016 update
- WHO – Treatment guidelines for isoniazid-resistant tuberculosis. Supplement 2018
- WHO – Xpert MTB/RIF system for the diagnosis of pulmonary and extra pulmonary TB in adults and children. Policy update. WHO 2013.
- Wilkinson RJ et al. Tuberculosis meningitis. Nature Reviews, Neurology 2017; 13:581-598
- Xavier Blanc F, Sok T, Laureillard D, Torand L, Rekacewicz D, Nerrienet E, et al. for the CAMELIA (ANRS 1295-CIPRA KH001) Study Team. Earlier versus later start of antiretroviral therapy in HIV infected adults with tuberculosis. N Engl J Med. 2011;365:1471–81. [PMC4879711]
CLINICAL CASES
Tropical Travel Trouble
Peer Reviewers
• Dr McBrideID physician, Wisconsin
Dr Neil Long BMBS FACEM FRCEM FRCPC. Emergency Physician at Kelowna hospital, British Columbia. Loves the misery of alpine climbing and working in austere environments (namely tertiary trauma centres). Supporter of FOAMed, lifelong education and trying to find that elusive peak performance.