Liddle Syndrome
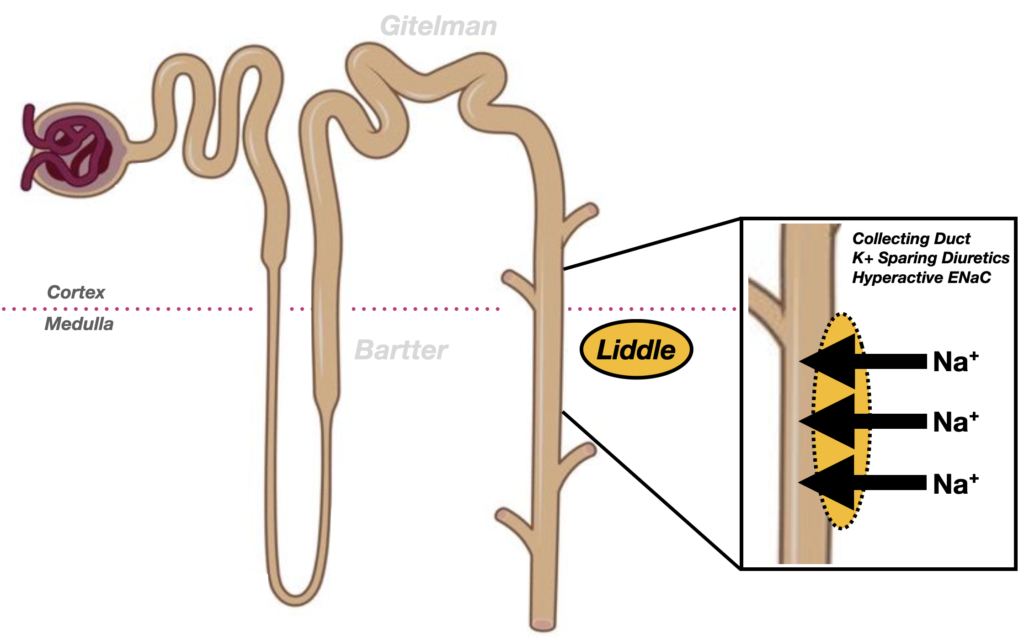
Liddle syndrome affects the collecting duct, where a gain-of-function mutation in the epithelial sodium channel (ENaC) leads to constitutive sodium reabsorption. This results in sodium retention, hypokalaemia, metabolic alkalosis, suppressed renin, and low aldosterone, producing a phenotype that mimics mineralocorticoid excess despite absent hormonal overproduction.
Liddle syndrome, also called pseudohyperaldosteronism, is a rare autosomal dominant disorder of salt handling that results in early-onset, resistant hypertension, hypokaleamia, and metabolic alkalosis. It is pathophysiologically characterised by low plasma renin activity and suppressed aldosterone secretion, mimicking mineralocorticoid excess.
The syndrome arises from mutations in the epithelial sodium channel (ENaC) of the distal nephron, leading to constitutive sodium reabsorption. Treatment with ENaC inhibitors like amiloride is effective; mineralocorticoid antagonists are ineffective.
History
1963 – Original description by Grant Winder Liddle (1921-1989), Bledsoe and Coppage who published “A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion.”
We describe a familial disorder in which several members developed severe hypertension, hypokalemia, and metabolic alkalosis. These features mimic primary aldosteronism, yet plasma aldosterone concentrations were negligible. The condition was inherited in a dominant fashion, and did not respond to adrenalectomy.
This paper identified a familial disorder of renal sodium handling unresponsive to usual therapies for hyperaldosteronism. Liddle proposed a primary defect in sodium transport in the distal nephron before molecular techniques could confirm it.
1995 – Genetic Confirmation by Hansson JH et al. in Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Identified mutations in the SCNN1G gene encoding the γ-subunit of ENaC, resulting in impaired degradation and persistent activity of sodium channels. This study provided molecular confirmation of Liddle’s 1963 hypothesis.
1990s–2000s – Further mutations identified in SCNN1B and SCNN1A. Affected ENaC subunits fail to interact with ubiquitin ligase Nedd4 due to PY motif mutations, preventing internalization and degradation.
Treatment refinement – Amiloride and triamterene, which directly block ENaC, became the mainstay of therapy. Spironolactone is ineffective due to aldosterone independence.
2010s–2020s – Expanded phenotypic spectrum recognized. Some patients present normokalaemic or late in adulthood. Over 30 distinct mutations now documented across more than 70 families worldwide.
Comparative Context:
Comparison of Bartter, Gitelman and Liddle syndromes
| Feature | Bartter Syndrome | Gitelman Syndrome | Liddle Syndrome |
|---|---|---|---|
| Defect location | Thick Ascending Limb of Loop of Henle | Distal Convoluted Tubule | Collecting Duct (ENaC channel) |
| Transporter affected | NKCC2 (Na⁺-K⁺-2Cl⁻ cotransporter) | Na⁺-Cl⁻ cotransporter (SLC12A3) | Epithelial Na⁺ Channel (ENaC; SCNN1B/SCNN1G) |
| Pathophysiologic mimic | Loop diuretics (lose Ca²⁺) | Thiazide diuretics (preserve Ca²⁺) | Aldosterone excess (but low aldosterone) |
| Serum potassium (K⁺) | ↓ Hypokalaemia | ↓ Hypokalaemia | ↓ Hypokalaemia |
| Serum bicarbonate (HCO₃⁻) | ↑ Metabolic alkalosis | ↑ Metabolic alkalosis | ↑ Metabolic alkalosis |
| Serum magnesium (Mg²⁺) | Normal or mildly ↓ | ↓ Hypomagnesemia | Normal |
| Urinary calcium | ↑ Hypercalciuria | ↓ Hypocalciuria | Normal |
| Blood pressure | Normal or low | Normal or low | ↑ Hypertension |
| Renin | ↑ Elevated | ↑ Elevated | ↓ Suppressed |
| Aldosterone | ↑ Elevated | ↑ Elevated | ↓ Suppressed |
| Age of onset | Neonatal/Childhood | Childhood/Adolescence | Childhood/Adolescence |
| Response to treatment | NSAIDs (↓ prostaglandins), K⁺, spironolactone | Mg²⁺ and K⁺ supplementation, ± NSAIDs | Amiloride or triamterene (ENaC inhibitors) |
Associated Persons
- Frederic Crosby Bartter (1914-1983) | Bartter Syndrome
- Grant Winder Liddle (1921-1989) | Liddle Syndrome
- Hillel Jonathan Gitelman (1932-2015) | Gitelman Syndrome
References
Historical references
- Liddle GW, Bledsoe T, Coppage WS. A familial renal disorder stimulating primary aldosteronism, but with negligible aldosterone secretion. Trans Assoc Am Phys 1963; 76: 199-213
Eponymous term review
- Botero-Velez M, Curtis JJ, Warnock DG. Brief report: Liddle’s syndrome revisited–a disorder of sodium reabsorption in the distal tubule. N Engl J Med. 1994 Jan 20;330(3):178-81.
- Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet. 1995 Sep;11(1):76-82.
- Awadalla M, Patwardhan M, Alsamsam A, Imran N. Management of Liddle Syndrome in Pregnancy: A Case Report and Literature Review. Case Rep Obstet Gynecol. 2017;2017:6279460.
- Enslow BT, Stockand JD, Berman JM. Liddle’s syndrome mechanisms, diagnosis and management. Integr Blood Press Control. 2019 Sep 3;12:13-22.
eponymictionary
the names behind the name
BMedSci (Pharm) MB ChB, Edinburgh University. Emergency and Internal Medicine training. Interested in neuropharmacology and electrophysiology
BA MA (Oxon) MBChB (Edin) FACEM FFSEM. Emergency physician, Sir Charles Gairdner Hospital. Passion for rugby; medical history; medical education; and asynchronous learning #FOAMed evangelist. Co-founder and CTO of Life in the Fast lane | On Call: Principles and Protocol 4e| Eponyms | Books |

