Valproate: Still Big Trouble!
aka Toxicology Conundrum 056
This case-based Q&A follows on from Toxicology Conundrum 055, let’s recap:
A 35 year-old male is brought in by ambulance after being discovered by friends in a semi-comatose state and surrounded by empty pill packets. It is estimated he has ingested 96g of enteric-coated sodium valproate, sometime in the last 12 hours.
On arrival to the emergency department he has:
- a patent airway
- RR 16, SaO2 99% on 6L oxygen, and right mid zone crackles on auscultation of the lungs
- HR of 106/min, BP of 123/76, with warm peripheries
- GCS is 8 (E2V2M4)
Venous blood gas on arrival showed a pH of 7.29, PCO2 of 46, HCO3 of 23 and a lactate of 5.3.
Initial VPA level came back as 889mg/L (6224 micromol/L), and initial biochemistry showed:
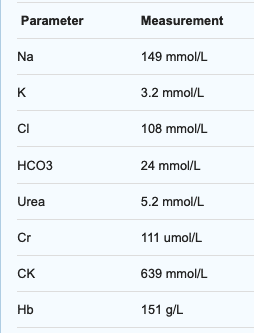
There was no evidence of trauma or rhabdomyolysis/compartment syndrome. The patient was intubated in the ED for decreasing GCS and airway protection. CXR following intubation shows infiltrate in the right mid zone consistent with aspiration. Propofol is running at 20mg/hr, and oxygenation/ventilation is adequate on pressure support. He had two intravenous cannulae, a nasogastric tube, an arterial line and an IDC inserted.
The toxicology service reviewed the patient in the ED and was concerned that this patient is high risk for multi-organ failure given his ingested dose of >1000mg/kg and serum VPA level of 889mg/L which puts him at risk of multi-organ failure. They advised that the patient would benefit from whole bowel irrigation and would like the ICU staff to commence this with 2L of bowel prep solution in the first hour, and 1L every hour after that, with auscultation of bowel sounds every 30minutes to check for ileus.
Questions
Q1. What investigations may help guide further management in this case?
Answer and interpretation
The following may help
- UEC — assessing for hypernatraemia, developing renal impairment, hypocalcemia
- CK — worsening rhabdomyolysis from prolonged immobility
- Blood gas — 1-2 hourly depending on haemodynamics and clinical condition. Assessing for metabolic (lactic) acidosis and hypoglycemia
- Sodium valproate levels — 4-6hourly intervals initially to determine whether they are rising or falling. Remember:
- <450mg/L: limited toxicity
- 450 – 850mg/L: moderate to severe toxicity
- >850mg/L: greatest risk for serious or life-threatening effects including coma, respiratory depression, metabolic acidosis, hypotension
- Ammonia levels: may be helpful in predicting presence of underlying encephalopathy and/or development of cerebral oedema in patients who are mechanically ventilated and sedated (Clay and Hainline, 2007)
The ICU resident now wants to know: Are we going to dialyse this patient?
Q2. What is the role for renal replacement therapy in this case?
Answer and interpretation
The decision to institute RRT to enhance elimination of VPA overdose should be made after review of the clinical picture and serum VPA concentrations, and should be considered for any patient with high serum VPA concentrations (particularly >850mg/ml) and hemodynamic instability or multi organ impairment.
VPA protein binding becomes saturated with overdose meaning there may be more free valproate accessible to removal from the circulation. Protein binding varies accoridng to the level:
- 90% at therapeutic levels
- 54-70% at 150mg/L
- 35% at >300mg/L
- and <10% at >500mg/L
which combined with its low Vd, low molecular weight, and water solubility make VPA an ideal agent for removal by hemodialysis (Licari et al, 2009)
Case reports and series document the use of haemodialysis (HD), haemoperfusion (HP), continuous venovenous haemodiafiltration (CVVHDF), and sustained low-efficiency dialysis with filtration (SLEDD-f) (Thanacoody, 2009):
- Clearance rates of up to 140ml/min of VPA have been calculated during HD for overdose presentations (endogenous clearance estimated at 5-30ml/min in therapeutic dosing), with corresponding reductions in VPA half-life, and rapid improvement in clinical parameters, particularly neurologic and haemodynamics
- It appears haemodialysis is an effective method of enhancing VPA removal at high serum concentrations, with benefit decreasing as you approach therapeutic serum concentrations
A breakdown of the evidence for each modality includes:
- IHD (see also Kielstein et al, 2003)
- preferred modality, effective at removing VPA but can be limited by haemodynamic instability
- Rebound levels after cessation have been reported, so multiple sessions may be required
- HP
- also effective, but probably less so than IHD as a result of the charcoal column becoming saturated relatively early
- Also associated with thrombocytopenia, requires use of heparin, and does not allow concurrent correction of electrolyte or acid base disturbances
- Not routinely used any longer (Thanacoody, 2009)
- CVVHDF
- slower rate of removal of VPA, may not be adequate as an initial extracorporeal removal method in patients with large ingestions/VPA concentrations (Thanacoody, 2009)
- Better tolerated by patients who are haemodynamically unstable
- SLEDD-f
- one case report, by Khan et al (2008), exists for this “hybrid” type of dialysis
- the patient had a peak VPA level of 1700mg/L, which fell to approximately 500mg/L after 16hrs of SLEDD-f
- There was no rebound in VPA serum concentrations seen following cessation of the single session
So, does this patient need dialysis?
The patient underwent whole bowel irrigation for 4 hours using a closed rectal rube system, and tablet residue was identified in the effluent. He remained haemodynamically stable with an adequate urine output and renal function in the first 24 hours with falling valproate levels, so the decision was made to not dialyse.
Here are the serial Valproate levels (mg/L):
- Admission (0 hours) 889
- 4 hours 638
- 8 hours 509
- 12 hours 448
- 18 hours 269
- 24 hours 163
Over 3 days the patient’s aspiration pneumonia improved and ventilation was weaned to extubatable parameters by day 3. However, despite minimal sedation with propofol, the patient failed to wake when sedation was ceased. A CT scan was performed but did not show any abnormality.
Q3. What is the likely cause of his decreased GCS in view of VPA overdose?
Answer and interpretation
This is probably valproic acid induced hyperammonia encephalopathy (VHE)
- VHE has been reported secondary to acute VPA overdose and in chronic normal therapeutic dosing, presenting as an acute and profound decreased level of consciousness, with or without increased seizure activity
- this is usually in association with an increased serum ammonia level (Mock and Schwetschenau, 2008)
- Patients at higher risk for VHE include those with liver impairment, on multiple anticonvulsants, or have other causes of increased ammonia production.
Carnitine deficiency occurs in VPA use, and is thought to be ultimately responsible for increased ammonia production.
Q4. What are the treatment options for the decreased GCS?
Answer and interpretation
L-carnitine supplementation
- Treatment involving L-carnitine supplementation to correct carnitine deficiency might help prevent the accumulation of ammonia
- Evidence consists largely of case reports and retrospective case series demonstrating benefits with L-carnitine use
- Further research is needed to better define its role and regime of use in VPA toxicity (Lheureux and Hantson, 2009)
Haemodialysis
- reported as a treatment used in VHE, but clear benefit is yet to be established
- RRT may be of benefit early in very large ingestions with high VPA levels to reduce the incidence of neurotoxic effects, through enhanced clearance of VPA and possibly via the enhanced clearance of ammonia (Thanacoody, 2009)
- Licari et al (2009) describes two approaches to the treatment of two almost identical presentations of VPA overdose, both of which ingested 16g, and were haemodynamically stable throughout admission 10:
- Patient 1:
Peak level 595mg/L, intubated and ventilated, and managed conservatively with no RRT. Patient had protracted coma, high ammonia levels, cerebral oedema on CT, and developed seizures on day 3 of admission. CVVHF was then commenced, with corresponding decrease in VPA and ammonia levels, and recovery over a period of 7 days, being discharged on day 11 - Patient 2:
Peak level 795mg/L, Early institution of IHD for 6hrs on admission to ICU, then CVVHDF overnight, and a further HD session of 9 hours on day 2. VPA and ammonia levels recovered to normal and she was discharged to the ward on day 3
- Patient 1:
Time and meticulous supportive care!
Over the next 10 days the patient has ongoing, but improving, decreased level of consciousness. The biochemistry and VPA level progression is included below.
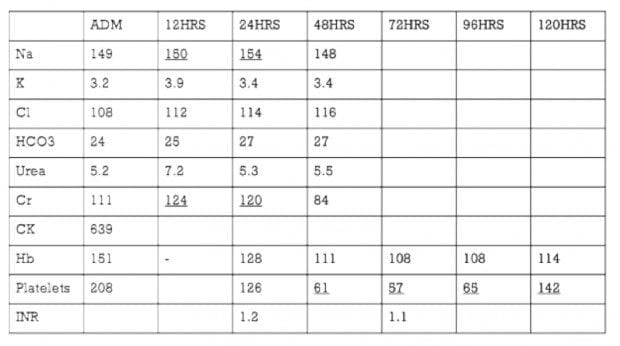
By day 11 post-admission he had improved sufficiently to allow discharge to a mental health facility.
Q5. Describe and interpret the abnormalities in the biochemistry.
Answer and interpretation
This shows:
- hypernatraemia due to high sodium load from a large ingestion of sodium valproate
- thrombocytopaenia — reported incidence of 8% in case series (Spiller et al, 2000)
References and Links
- Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest. 2007;132(4):1368–78.
- Licari E, Calzavacca P, Warrillow SJ, Bellomo R. Life-threatening sodium valproate overdose: a comparison of two approaches to treatment. Crit Care Med. 2009;37(12):3161–4.
- Thanacoody RHK. Extracorporeal elimination in acute valproic acid poisoning. Clin Toxicol (Phila). 2009;47(7):609–16..
- Kielstein JT, Woywodt A, Schumann G, Haller H, Fliser D. Efficiency of high-flux hemodialysis in the treatment of valproic acid intoxication. J Toxicol Clin Toxicol. 2003;41(6):873–6.
- Khan E, Huggan P, Celi L, MacGinley R, Schollum J, Walker R. Sustained low-efficiency dialysis with filtration (SLEDD-f) in the management of acute sodium valproate intoxication. Hemodial Int. 2008;12(2):211–4
- Dharnidharka VR, Fennell RS, Richard GA. Extracorporeal removal of toxic valproic acid levels in children. Pediatr Nephrol. 2002;17(5):312–5.
- Hicks LK, McFarlane PA. Valproic acid overdose and haemodialysis. Nephrol Dial Transplant. 2001;16(7):1483–6.
- Mock CM, Schwetschenau KH. Levocarnitine for valproic-acid-induced hyperammonemic encephalopathy. Am J Health Syst Pharm. 2012;69(1):35–9
- Lheureux P, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol. 2009;(2009):101–111
- Spiller HA et al. Multicenter case series of valproic acid ingestion: serum concentrations and toxicity. J Toxicol Clin Toxicol. 2000;38(7):755–60.

CLINICAL CASES
Toxicology Conundrum
Dr Neil Long BMBS FACEM FRCEM FRCPC. Emergency Physician at Kelowna hospital, British Columbia. Loves the misery of alpine climbing and working in austere environments (namely tertiary trauma centres). Supporter of FOAMed, lifelong education and trying to find that elusive peak performance.
