Valproate: Big Dose, Big Trouble!
aka Toxicology Conundrum 055
A 35 year-old male is brought in by ambulance after being discovered by friends in a semi-comatose state and surrounded by empty pill packets. It is estimated he has ingested 96g of enteric-coated sodium valproate, sometime in the last 12 hours.
On arrival to the emergency department he has:
- a patent airway
- RR 16, SaO2 99% on 6L oxygen, and right mid zone crackles on auscultation of the lungs
- HR of 106/min, BP of 123/76, with warm peripheries
- GCS is 8 (E2V2M4)
Venous blood gas on arrival shows a pH of 7.29, PCO2 of 46, HCO3 of 23 and a lactate of 5.3.
Questions
Q1. By what mechanism does sodium valproate or valproic acid (VPA) work?
Answer and interpretation
VPA’s mechanism of action is not fully understood.
- Blockade of voltage dependent Na+ channels and increased brain levels of GABA are possibly the result of inhibition of GABA degradative enzymes or inhibition of GABA reuptake by neuronal cells, and are though to contribute to its anticonvulsant effect.
- VPA appears to have no significant effects on the autonomic nervous system, blood pressure, renal function, or body temperature during therapeutic levels of administration.
VPA use is cautioned in, and has been associated with, pancreatitis, hepatic dysfunction, and hyperammonia during therapeutic use. Increased VPA levels can occur if administered with antipsychotic agents or antidepressants (MAOI, TCA, SSRI)
Q2. Describe the toxicokinetics of VPA
Answer and interpretation
Absorption
- Almost completely absorbed with peak serum concentrations of 1 – 4 hours (plain tablets/syrup), and 3-7 hours (enteric coated tablets)
- Peak levels can be delayed up to 18 hours in overdose
- In very large ingestions particularly of enteric-coated formulations, formation of pharmacobezoars can significantly alter and delay the absorption kinetics of VPA
Distribution
- Rapid distribution, small Vd (0.1-0.4L/kg)
- VPA displays nonlinear kinetics as a result of concentration dependent plasma protein binding and a short half-life
- At therapeutic levels there is 90% plasma protein binding (60% to albumin) BUT in presence of high levels (>120mg/L) or low serum albumin, binding sites become saturated, and result in higher concentration of free drug
- It is estimated that at 150mg/L, 54-70% of VPA is protein bound; >300mg/L, 35% is protein bound; >500mg/L possibly <10% is protein bound (Licari et al, 2009)
Metabolism
- Hepatic via glucuonidation (40-60%), beta-oxidation (30-40%), and omega-oxidation (fraction), producing active metabolites
- A small amount is excreted unchanged in urine (1-3%)
- Overdose or supra-therapeutic dosing result in increased toxic metabolite production and depletion of carnitine required for beta oxidation metabolism
- Metabolites are implicated in hepatotoxicity (4-EN-VPA); omega oxidation), cerebral oedema (2-EN-VPA; beta oxidation), and precipitation of hyperammonia (propionic acid; omega oxidation) (Lhereux et al, 2005 and Lhereux et al, 2009)
Elimination
- Renal after extensive metabolism
- Plasma half-life range is 4-16hrs, and can be prolonged to >30hours in overdose
Q3. What are the clinical features in VPA overdose?
Answer and interpretation
In large doses or overdosage, multi-organ system effects are thought to be the result of the influence of increased GABA and interference with metabolic pathways.
CNS effects
- CNS depression is the most common symptom, with coma almost universal in patients with drug levels >850mg/L ref5. This may persist despite normalisation of VPA serum concentrations, possibly related to toxic metabolites implicated in CNS toxicity having prolonged half-lives ref6
- Cerebral oedema, presenting up to 72 hours post ingestion, can occur (probably due to hyperammonemia and/or the neurotoxic metabolite 2-en-VPA ref7,8
Cardiovascular
- Tachycardia (seen in 17% of all patients presenting with VPA overdose in a case series) ref5
- Hemodynamic instability (~25% of those with levels >850mg/L) ref5
Gastrointestinal
- Nausea, vomiting and abdominal pain in overdose ref9
- Pancreatitis and hepatotoxicity can occur but are rarely associated with toxicity ref7
Metabolic
- Hypernatraemia (secondary to “sodium” load delivered via in sodium valproate overdose)
- high anion gap metabolic acidosis (HAGMA)
- lactic acidosis
- hypocalcaemia
- hypoglycaemia
- hyperammonemia
Q4. What is the risk assessment based on the ingested dose?
Answer and interpretation
Severity of toxicity is related to the dosage ingested (Murray et al, 2010):
- <200mg/kg: nil to minor effects, i.e. mild drowsiness
- 200 – 400mg/kg: moderate toxicity, i.e. CNS depression
- 400 — 1000 mg/kg: severe toxicity, i.e. Coma
- >1g/kg: potentially life threatening, i.e. profound coma, multi-organ toxicity including hypotension, metabolic (lactic) acidosis, cerebral oedema, biochemical abnormalities, bone marrow suppression
This patient has ingested a life-threatening dose.
Q5. What is the significance of VPA serum concentrations?
Answer and interpretation
VPA concentrations are available in most hospital laboratories. The normal therapeutic range is 50-100mg/L (350-700micromol/L). Concentrations greater than 850mg/L (6000micromol/L) are associated with severe poisoning .ref5
Correlation of Clinical Effects with VPA levels:
- <450mg/L: limited toxicity
- 450 – 850mg/L: moderate to severe toxicity
- >850mg/L: greatest risk for serious or life-threatening effects including coma, respiratory depression, metabolic acidosis, hypotension
There have been cases of inaccurate valproate level measurements in severe overdoses because samples were not diluted before measurement — write on the laboratory order form suspected valproate overdose and the likely ingested dose so that laboratory staff are aware.
Q6. Given the presentation and the information regarding VPA overdose, how would you manage this patient at this point?
Answer and interpretation
Resuscitation
- Most importantly, this patient requires ABCDE simultaneous assessment and resuscitation. Points to consider in this ingestion include:
- Airway and Breathing — airway protection is compromised by reduced GCS, and given the ingested dose, this is unlikely to resolve in the near future. The patient may have already aspirated based on the chest signs. Intubation and ventilation is required.
- Circulation — the patient is mildly tachycardic, but has an adequate BP at this stage; adequate IV access is required in preparation for fluids and inotropic support as required; IDC to monitor urine output
- Disability — sedation requirements likely to be minimal given CNS effects associated with this dosage. Monitor for evidence of raised ICP due to cerebral edema
- Head to toe examination — for evidence of trauma/falls which may have occurred prior to coma, and rhabdomyolysis/compartment syndrome resulting in hyperkalemia from prolonged immobility
Risk assessment
- see Q4
- obtain a corroborative history and assessment of likely ingested dose through examination of empty pill packets, discussion with family, friends and external health practitioners to confirm the risk assessment if possible
Supportive care and monitoring
- appropriate FASTHUGS IN BED Please
Investigations
- UEC, LFT, FBC, CMP and CK
- Blood gas for K, gas exchange, lactate, HAGMA
- Valproate serum concentration
Decontamination
- see Q7
Disposition
- arrange ICU admission
- will need psychiatric review prior to discharge from hospital if the patient survives
Q7. What methods if any would you consider for decontamination in this case?
Answer and interpretation
This patient has ingested a large amount of a sustained release preparation of sodium valproate, with potential for multi-organ involvement or failure, with an unknown time of ingestion.
Potential benefits from reduced adsorption and/or reduced peak concentrations are particularly important in life threatening ingestions of slow release substances that can cause overwhelming organ failure, do not have an available antidote or have limited treatment options.
Decontamination options are:
- single dose activated charcoal (SDAC)
- multi-dose activated charcoal (MDAC)
- whole bowel irrigation (WBI)
Gastric lavage and induced emesis are no longer routinely used, have unreliable toxin removal, and risks that outweigh any benefit.
The position statements of the American Academy of Clinical Toxicology (AACT) suggests the following when applied to large VPA overdose (ref 12 to 14)
- SDAC
- Should not be administered as a routine intervention
- It can be considered up to an hour post ingestion (4 hours in slow or enteric release preparations) in those who have ingested potentially toxic dose, and who have an intact, protected airway
- Usual dose is 1gm/kg
- MDAC
- Reported to be used in multiple cases of VPA overdose, but AACT position statement states that there is no evidence that MDAC increases the elimination of VPA, and should only be considered in specific overdoses (carbemazepine, dapsone, phenobarbitone, quinine, or theophylline)
- it may aid decontamination (rather than enhanced elimination) given the slow absorption of valproate
- WBI
- consider in sustained release or enteric-coated ingestions that are potentially toxic or life threatening in patients presenting greater than two hours.
- Contraindications include bowel obstruction, perforation, ilieus, or those with unprotected airways and/or hemodynamic instability.
- WBI is often considered labour intensive, and just generally unpleasant and messy, but the availability of closed rectal tube systems in ICU environments mean that the procedure is now more “user friendly”
- While theoretical benefits exist for WBI, it must be noted that there is little evidence to support its use and these must be weighed against potential harms
- Preparations containing macrogol 3350 powder with electrolytes (i.e. ColonLYTELY) can be used
- Suggested dosage is 2L via NGT in first hour, followed by 1L per hour ref9
- Close monitoring of bowel sounds is necessary, and if ileus is suspected, WBI should cease immediately.
WBI may be the only option with a chance of providing some benefit, provided the patient does not have any contraindications (and we can convince those who will be administering it that it is worthwhile!). This therapy is controversial and its use is best discussed with a clinical toxicologist.
Initial VPA level comes back as 889mg/L (6224 micromol/L).
Initial biochemistry includes:
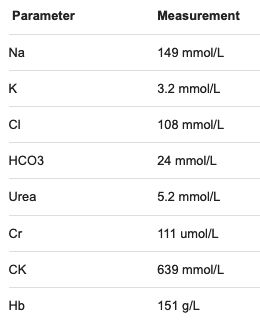
Q8. What is the risk assessment for this patient now?
Answer and interpretation
This patient is at risk of severe toxicity.
- He has potentially ingested >1000mg/kg at an unknown time, and has a VPA level >850mg/L.
- Prolonged coma, hemodynamic instability and metabolic derangement are possible if not probable.
This patient needs to be closely monitored, admitted to an ICU, and have serial VPA and biochemistry testing.
This case will continue in a follow up post shortly…as Valproate: Still Big Trouble!
References
- Licari E, Calzavacca P, Warrillow SJ, Bellomo R. Life-threatening sodium valproate overdose: a comparison of two approaches to treatment. Crit Care Med. 2009;37(12):3161–4.
- Thanacoody RHK. Extracorporeal elimination in acute valproic acid poisoning. Clin Toxicol (Phila). 2009;47(7):609–16.
- Lheureux P, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol. 2009;(2009):101–111
- Spiller HA et al. Multicenter case series of valproic acid ingestion: serum concentrations and toxicity. J Toxicol Clin Toxicol. 2000;38(7):755–60.
- Sztajnkrycer MD. Valproic acid toxicity: Overview and management. Journal of Toxicology – Clinical Toxicology 2002; 40(6):789-801.
- Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest. 2007;132(4):1368–78.
- Hicks LK, McFarlane PA. Valproic acid overdose and haemodialysis. Nephrol Dial Transplant. 2001;16(7):1483–6.

CLINICAL CASES
Toxicology Conundrum
Dr Neil Long BMBS FACEM FRCEM FRCPC. Emergency Physician at Kelowna hospital, British Columbia. Loves the misery of alpine climbing and working in austere environments (namely tertiary trauma centres). Supporter of FOAMed, lifelong education and trying to find that elusive peak performance.
