Anti-NMDA Receptor Encephalitis
Anti-NMDA receptor encephalitis is a severe, multistage, but treatable disorder presenting with prominent features of psychosis.
The presence of pronounced psychiatric symptoms often results in a misdiagnosis of functional psychosis or schizophrenia.
This disease is most common in reproductive-aged (<45 years) females. There is a high association with ovarian teratoma (~50% of cases in patients >18 years).
Diagnosis is vital as the condition, if untreated, can lead to severe permanent neurological deficits or death. Early recognition is paramount as outcomes improve with early treatment. Diagnosis is often delayed due to its relative rarity and the prominence of psychiatric symptoms.
Diagnosis
- Detection of GluN1 or GluN2 antibodies in CSF.
- Characteristic clinical picture.
Specific Management
- High-dose steroids.
- Intravenous immunoglobulin (IVIG).
- Plasma exchange.
- Immunosuppressants.
- Resection of underlying neoplasm.
Outcomes
- ~45% full recovery.
- ~30% mild stable deficits.
- ~20% severe deficits.
- ~5% mortality.
History
First identified by Dr Josep Dalmau and colleagues at the University of Pennsylvania in 2007.
Epidemiology
- Most common in females <45 years.
- ~80% of cases are female.
- Mean patient age: 23 years (range 5–76).
- ~50% of patients >18 years have associated ovarian teratoma.
- Can occur in children, males, and the elderly.
Physiology
NMDA Receptor
- N-methyl-D-aspartate receptor (NMDAR): glutamate/glycine receptor and ion channel protein in central neurones.
- One of four ionotropic glutamate receptors:
- AMPA.
- Delta.
- Kainate.
- NMDA.
Structure
- Heterotetramer of:
- 2 GluN1 subunits.
- 2 GluN2 subunits.
Function
- Requires glutamate + glycine for activation.
- Key role in synaptic plasticity and memory.
- Especially prevalent in limbic system and hippocampus.
Pathophysiology
Encephalitis Types
- Infectious (see separate Encephalitis document).
- Autoimmune:
- Paraneoplastic encephalitis: Antibodies to intracellular neuronal proteins; invariably cancer-related.
- Autoimmune encephalitis: Antibodies to neuronal cell surface/synaptic proteins; may occur with or without cancer.
Anti-NMDAR Encephalitis
- Antibodies bind NMDA receptors → receptor internalisation → loss of function.
- May occur with or without ovarian teratoma.
- Possible mechanisms:
- Molecular mimicry from teratoma expressing NMDA receptors.
- Post-infectious (e.g. Herpes simplex virus).
Antibody Positivity
- Not all patients with NMDA receptor antibodies develop encephalitis.
- Incidental antibody positivity seen in other disease states.
Immunological Triggers
Association with Teratoma
- ~50% of adult female cases linked to ovarian teratoma.
- Tumour neural tissue may trigger immune response via antigen presentation.
Other Tumours
- Less common: lung, thymoma, breast, testicular cancers.
Association with HSV
- ~20% of patients with Herpes simplex encephalitis develop NMDA receptor antibodies.
Clinical Features
Progression
- Progressive, rapidly worsening neuropsychiatric symptoms are a strong diagnostic clue.
- Often follows predictable phases:
Phase 1: Prodrome
(~70% of patients)
- Headache.
- Lethargy/malaise.
- Fever.
- Myalgias.
- Nausea/vomiting.
Phase 2: Acute Neuropsychiatric Illness
Psychiatric Symptoms
- Anxiety/fear.
- Agitation/restlessness.
- Mood lability.
- Personality changes.
- Psychotic features:
- Delusions.
- Hallucinations.
- Disorganised thought.
- Bizarre behaviours.
- Speech disturbances:
- Paucity.
- Mutism.
- Echolalia.
- Short-term memory deficits/confusion.
Neurological Deficits
- Global altered consciousness:
- Decreased responsiveness.
- Catatonic-like states.
- Extrapyramidal movement disorders:
- Orofacial dyskinesias.
- Dystonic posturing.
- Chorea.
- Muscle rigidity.
- Autonomic instability:
- Hyperthermia (may mimic NMS).
- Bradycardia/tachycardia.
- Hypotension/hypertension.
- Seizures (partial motor or complex).
- Hypoventilation:
- May require prolonged ventilation.
Phase 3: Recovery / Relapse
- Prolonged course but may show spontaneous improvement.
- Hospitalisation often ~3–4 months.
- Autonomic/respiratory recovery first.
- Cognitive/psychiatric recovery slowest.
Phase 4: Persistent / Permanent Deficits
- Deficits in executive/cognitive function.
- Behavioural abnormalities.
- Abnormal sleep patterns.
Prognosis
- Without treatment: deterioration and death possible.
- With treatment:
- ~45% full recovery.
- ~30% mild stable deficits.
- ~20% severe deficits.
- ~5% mortality.
- Relapse rate ~25%.
Differential Diagnoses
- Primary psychiatric illness.
- Other causes of encephalitis:
- Infectious.
- Other autoimmune.
- Toxic drug reactions:
- Neuroleptic malignant syndrome.
Investigations
Diagnosis
- Detection of GluN1 or GluN2 antibodies in CSF + clinical picture.
Blood Tests
- FBE.
- CRP.
- U&E / glucose.
- Ca / Mg / PO4.
- LFTs.
- TFTs.
Urine Drug Screen
- Consider if drug toxicity suspected.
CSF
- Detection of GluN1 / GluN2 IgG antibodies → diagnostic.
- CSF findings:
- Lymphocytic pleocytosis.
- Oligoclonal bands (~60%).
- Elevated protein.
- CSF antibody titres correlate with clinical outcome better than serum.
EEG
- Abnormal in most cases:
- Slow, disorganised activity (delta/theta).
- Superimposed seizures.
Pelvic Ultrasound
- For detection of ovarian tumours.
CT Scan
- Initial work-up to exclude other causes.
- May detect ovarian teratoma.
MRI
- ~50% normal MRI.
- ~50% show:
- Bilateral T2 / FLAIR hyperintensities:
- Medial temporal lobe (hippocampus).
- Frontal cortex.
- Cerebellar cortex.
- Medulla oblongata.
- Spinal cord.
- Bilateral T2 / FLAIR hyperintensities:
- Hippocampal lesions = predictor of poor prognosis.
PET Scan
- Not routinely performed.
- May show increased frontal-occipital gradient of glucose metabolism.
Management
General Management
Supportive Care
- Hypoventilation may require prolonged ventilation.
Seizures
- Treated along conventional lines.
Psychiatric Symptoms
- Management is challenging.
- High-dose dopamine blockade may exacerbate dyskinesia/dystonia.
- Preferred agents:
- Anticholinergics.
- Benzodiazepines.
- Valproic acid.
- Sedating antipsychotics:
- Quetiapine.
- Chlorpromazine.
Specific Management
1. High-Dose Steroids
- IV methylprednisolone 1 g/day for 5 days.
2. Intravenous Immunoglobulin (IVIG)
- 400 mg/kg/day for 5 days.
3. Plasma Exchange
- Alternative to IVIG.
- Challenging in agitated or unstable patients.
4. Immunosuppressants
- Used if no tumour or insufficient response to steroids/IVIG.
- Options:
- Cyclophosphamide.
- Rituximab.
5. Resection of Neoplasm
- Essential if tumour identified.
Appendix 1: The NMDA Receptor
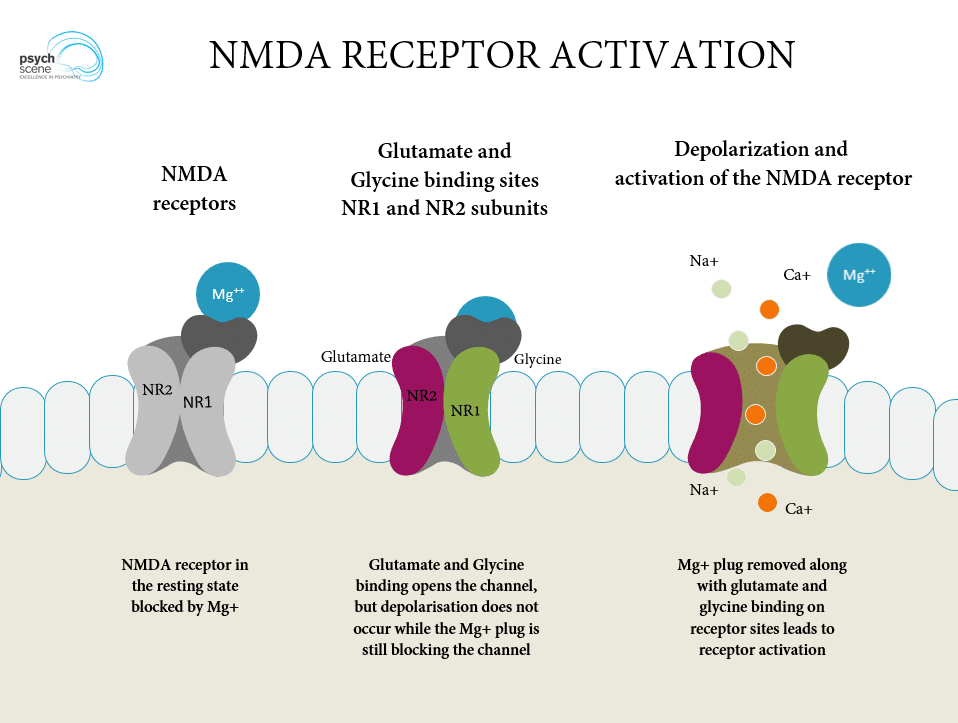
Left: NMDA receptor in the resting state, blocked with magnesium.
Middle: Glutamate binding to NR2 and glycine binding to NR1 results in ion channel opening.
Right: Mg is dislodged and sodium and calcium ions enter the neuron.
Image credit: Psych Scene Hub (Psych Scene) – used with permission
The possible role of the NMDA receptor in psychosis and schizophrenia:
- NMDA receptor antagonists, like phencyclidine, can mimic some of the symptoms of schizophrenia.
- NMDA receptor has thus been implicated in the pathogenesis of schizophrenia in both its “positive” and “negative” symptoms. .
- Normally, excitatory glutamate stimulates NMDA receptors in the interneuron resulting in GABA release from that neurone.
- GABA, in turn, inhibits the release of dopamine within mesolimbic pathways.
- Thus glutamatergic pathway can ultimately acts as a block on mesolimbic dopamine pathways.
- If NMDA receptors are blocked, then the tonic inhibition of mesolimbic dopamine pathways is also ultimately blocked. This leads to elevated dopamine levels in the mesolimbic system and can result in schizophrenia-like symptoms.
Appendix 2

Image credit: Psych Scene Hub (Psych Scene) – used with permission.
References
Publications
- Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007 Jan;61(1):25-36.
- Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008 Dec;7(12):1091-8.
- Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2010 Apr;257(4):509-17
- Wandinger KP, Saschenbrecker S, Stoecker W, Dalmau J. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. J Neuroimmunol. 2011 Feb;231(1-2):86-91
- Punja M, Pomerleau AC, Devlin JJ, Morgan BW, Schier JG, Schwartz MD. Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis: an etiology worth considering in the differential diagnosis of delirium. Clin Toxicol (Phila). 2013 Sep-Oct;51(8):794-7.
- Young PJ, Baker S, Cavazzoni E, Erickson SJ, Krishnan A, Kruger PS, Rashid AH, Wibrow BA. A case series of critically ill patients with anti- N-methyl-D-aspartate receptor encephalitis. Crit Care Resusc. 2013 Mar;15(1):8-14.
- Rege S. Anti-N-methyl-D-aspartate (Anti-NMDA) Receptor Encephalitis – A Synopsis. Psyche Scene Hub 2018.
FOAMed
- Nickson C. Anti-NMDA Receptor Encephalitis. CCC
- Nickson C. Medically cleared? LITFL
Fellowship Notes
BMedSci (Hons) in Physiology, MBChB, Edinburgh University. Foundation training in Aberdeen and now working in Sir Charles Gairdner Emergency Department in Perth. Career interest in Anaesthetics, ICU and rural practice. Outside interests in hiking, camping and running; all of which made more possible by life in Western Australia.
Educator, magister, munus exemplar, dicata in agro subitis medicina et discrimine cura | FFS |


