Barrett’s oesophagus
Barrett’s oesophagus is replacement of the distal oesophageal squamous lining by columnar epithelium with intestinal metaplasia (goblet cells) extending ≥1 cm above the GOJ, best documented endoscopically and by biopsy. Extent is reported with the Prague C&M criteria. Barrett’s is the recognised precursor of oesophageal adenocarcinoma, progressing through low- then high-grade dysplasia in a metaplasia→dysplasia→carcinoma sequence.
Epidemiology & risk
Community prevalence is generally <5% (≈2% in Western cohorts), with detection rising where endoscopy is common. Risk is higher with age, male sex, chronic GORD, central obesity, and smoking; past Helicobacter pylori infection appears protective.
Clinical presentation
Barrett’s itself is usually asymptomatic; patients typically present with GORD symptoms (or are found incidentally at endoscopy).
Investigations & diagnosis
Gold-standard diagnosis is OGD with systematic biopsies (Seattle protocol) to confirm intestinal metaplasia and assess dysplasia; report segment length with Prague C&M. Confirmation of any dysplasia by a second expert GI pathologist is recommended.
Pathogenesis
Long-standing acid/bile reflux injures squamous mucosa, driving columnar metaplasia that can acquire dysplasia and progress to adenocarcinoma over years—creating opportunities for risk-based screening and surveillance.
Management (summary)
- Reflux control: PPIs titrated to heal oesophagitis and control symptoms; fundoplication considered if medical therapy fails (does not clearly regress Barrett’s or alter cancer risk).
- Surveillance: Interval depends on segment length and dysplasia grade (e.g., 3–5 y if <3 cm; 2–3 y if ≥3 cm; shorter for dysplasia), with expert histology review.
- Endoscopic eradication therapy (EET): EMR for visible lesions (for staging and therapy), plus ablation (RFA most used) to eradicate residual Barrett’s; now first-line for HGD and selected T1a EAC within MDT pathways.
Cancer risk and the “epidemic”
For uncomplicated, non-dysplastic Barrett’s, contemporary population-based estimates suggest progression ≈1–3 per 1000 patient-years, with higher risk in long-segment disease and with dysplasia. Meanwhile, oesophageal adenocarcinoma incidence has risen sharply in many countries since ~1960–1990, with persistent male predominance.
History
1833 – Johann Friedrich Hermann Albers (1802–1865) published Über stechende Geschwüre der Speiseröhre und der Atemwege (On perforating ulcers of the oesophagus and the airways). Albers provided the first systematic account of penetrating oesophageal ulcers at post-mortem, often complicated by tracheo-bronchial fistulae, establishing ulcer disease of the oesophagus long before reflux-metaplasia was recognised.
…schrecklich endende Oesophagitis veranlasst, den Weg dieser Bildung nehmen und eine Öffnung zwischen Schlund und Luftröhre veranlassen…
Albers 1833
1884 – Sir Morell Mackenzie (1837–1892) provided an early clinical delineation of oesophagitis and its complications. Mackenzie defined acute oesophagitis as
Acute idiopathic inflammation of the mucous membrane of the oesophagus giving rise to extreme odyn[o]phagia, and often to aphagia. The disease is attended with some danger, but generally ends in resolution, and only in extremely ran cases terminates in ulcer, abscess, or gangrene
Mackenzie 1884
1906 – Wilder Tileston (1875-1969) described 3 cases of Peptic ulcer of the œsophagus highlighting acid injury at the cardia. Tileston emphasised:
…the close resemblance of the mucous membrane about the ulcer to that normally found in the stomach…the first requisite for the formation of the peptic ulcer of the oesophagus is an insufficiency of the cardia.
Tileson 1906
1946 – Philip Rowland Allison (1907-1974) publishes Peptic ulcer of the esophagus proposing an aetiological switch from congenital short oesophagus to reflux/hiatal hernia. He concludes that ulceration occurs “where there is … derangement of the mechanism of the cardia [and] acid gastric juice flows back easily into the lower end of the esophagus.”
1948 – Allison consolidates the reflux/hernia model arguing that a “short oesophagus” is acquired from fibrosis/hernia rather than truly congenital in his paper Peptic ulcer of the oesophagus (Thorax)
1950 – Norman Rupert Barrett (1903-1979) published Chronic Peptic Ulcer of the Oesophagus and ‘Oesophagitis‘. He drew a sharp distinction between reflux oesophagitis and what he believed were true congenital anomalies of the oesophagus.
Barrett described the oesophagus as “that part of the foregut, distal to the cricopharyngeal sphincter, which is lined by squamous epithelium”. Finding columnar mucosa in this region, he attributed it to a congenitally shortened oesophagus with stomach extending into the chest. He argued that many so-called “peptic ulcers of the oesophagus” were in fact ulcers of a stomach pouch drawn into the mediastinum by a congenitally short oesophagus.
I stress that the word ‘oesophagitis’ is now a blunderbuss term used to cover many different pathological lesions; it should always be qualified by a descriptive adjective such as ‘reflux oesophagitis’. I believe that reflux oesophagitis is common and that it can give rise to ulceration of the oesophagus and stricture formation
In contrast with this lesion is another which has always up till now been assumed to be identical with it, and which has generally been described by pathologists, as opposed to clinicians, under the heading of ‘peptic ulcer of the oesophagus’. I submit that most of these cases are in truth examples of congenital short oesophagus, in which there is neither general inflammation nor stricture formation, but in which a part of the stomach extends upwards into the mediastinum
Barrett 1950
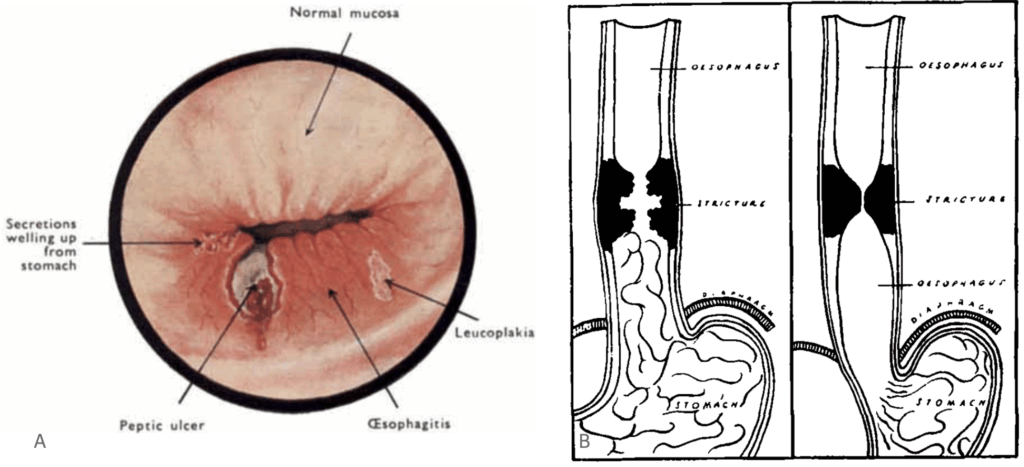
B. Diagram created by Barrett to demonstrate the ‘advancing’ gastric mucosa causing evidence of oesophagitis in the form of a stricture Barret, 1950
1952 – Basil C. Morson and John R. Belcher publish Adenocarcinoma of the oesophagus and ectopic gastric mucosa. The first clear report of oesophageal adenocarcinoma arising at the squamo–glandular junction within columnar lining. The authors emphasise adenocarcinoma of the oesophagus as a distinct entity.
A carcinoma had developed at the squamo-glandular junction….Adenocarcinoma of the oesophagus is an entity. It may arise from islands of ectopic gastric mucosa…
Morson, Belcher 1952
1953 – Allison and Alan Stewart Johnstone (1905–1990) reviewed cases of oesophagus lined with gastric mucous membrane, distinguishing reflux oesophagitis from true gastric-type ulcers within the oesophagus, and suggested the term “Barrett’s ulcer” for clarity.
…pathologists have been describing one thing and clinicians another, and they have had the same name. The clarification of this point has been so important, and the description of a gastric ulcer in the oesophagus so confusing, that it would seem to be justifiable to refer to the latter as Barrett’s ulcer. The use of the eponym does not imply agreement with Barrett’s description of an oesophagus lined with gastric mucous membrane as ‘stomach
Allison, Johnstone 1953
Allison and Johnstone provided a morphologic conclusion to identify the ‘organ’ involved in ulceration, but did not assert metaplastic transformation
…more careful examination… shows that it has no peritoneal covering, that the musculature is that of the normal oesophagus, that there may be islands of squamous epithelium within it, that there are no oxyntic cells in the mucosa, and that in addition to gastric glands there are present typical oesophageal mucous glands.
Allison, Johnstone 1953
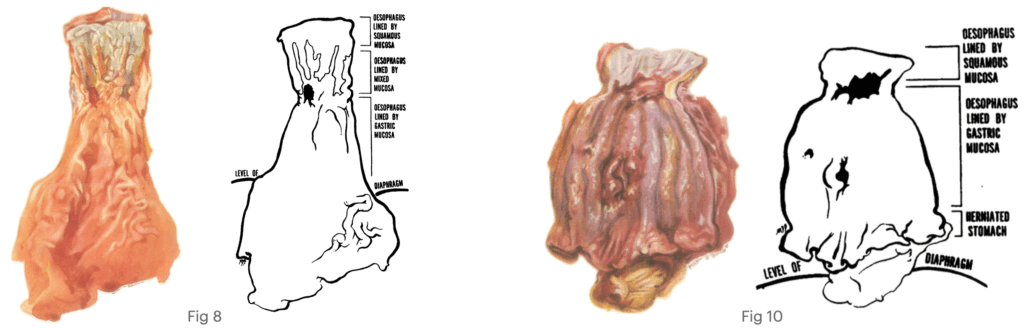
Fig. 10 Showing a reflux ulcer of the oesophagus with stenosis, Barrett’s ulcers of the gastric mucous membrane lining the oesophagus, and a small strip of normal gastric mucosa from the herniated stomach. Allison, Johnstone 1953
1957 – Barrett no longer insisted upon the congenital short oesophagus theory, publishing The lower esophagus lined by columnar epithelium acknowledging that the columnar-lined oesophagus represented a distinct clinical entity prone to ulceration and carcinoma.
1961 – John Isaac Hayward (1910-1999) published The lower end of the oesophagus defining the oesophagus as “a tube and all of this tube is oesophagus, regardless of its lining”. He introduced the term “junctional epithelium,” foreshadowing an acquired metaplastic model.
The oesophagus is a tube and all of this tube is oesophagus, regardless of its lining. The true junction is where the conducting tube changes to the digesting pouch. This means that the oesophagus is not lined exclusively by squamous epithelium. Its lower 1 to 2 cm. is lined by columnar epithelium, which also extends a little way into the stomach.
This epithelium has always been regarded as gastric in type and has been called cardiac epithelium, a rather absurd term which is saved from ambiguity because there happens to be no epithelium in the heart. Since, as will be shown below, it does not correspond to the cardia, I suggest that it be renamed “junctional epithelium.”
Hayward 1961
1970 – Bremner CG, Lynch VP, Ellis FH Jr created a canine reflux model which demonstrated columnar metaplasia under reflux, tipping consensus toward acquired origin (metaplasia→ dysplasia→ adenocarcinoma paradigm)
1976 – Paull et al. described The histologic spectrum of Barrett’s esophagus. They identified three epithelial phenotypes in the columnar-lined oesophagus (cardia-type; fundic-type; and intestinal/specialised with goblet cells). They linked the specialised type to cancer risk in subsequent work.
1983 – David B. Skinner et al. codified the ≥3 cm definition (historical practice) and highlighted malignant potential with reflux and dysplasia.
For the purposes of this study, Barrett’s esophagus is 3 or more cm of the distal tubular esophagus lined by columnar-type epithelium…The malignant degeneration of Barrett’s esophagus occurs in the presence of continuing reflux and persistent smoking.
Skinner 1983
1987 – In Barrett’s esophagus and adenocarcinoma, Brian J. Reid and Wilfred M. Weinstein outlined the now-accepted association and early risk estimates.
Barrett’s esophagus predisposes to the development of oesophageal adenocarcinoma…Retrospective analyses estimate a 30–40-fold increase in risk… one case of carcinoma in 175–441 patient-years.
Reid, Weinstein 1987
1994 – Stuart J. Spechler et al. in Prevalence of metaplasia at the gastro-oesophageal junction demonstrated 18% of general endoscopy patients had SCE at the GOJ (often <3 cm) and underscored why short segments matter for EAC epidemiology.
26 out of 142 patients (18%) were found to have specialised columnar epithelium… The groups did not differ in symptoms and endoscopic signs of reflux…Adults frequently have unrecognised segments of SCE at the gastro-oesophageal junction; this may underlie the rising frequency of cancer of the gastro-oesophageal junction.
Spechler 1994
2009 – Hejin P. Hahn et al. demonstrated intestinal differentiation markers (e.g., CDX2, DAS-1, villin) in non-goblet columnar epithelium, supporting a sequence from squamous → non-goblet → goblet metaplasia. Whilst Weitian Liu et al. showed DNA content abnormalities in non-goblet columnar epithelium similar to goblet-cell mucosa, arguing potential for neoplastic progression outside classic IM.
Associated Persons
- Norman Rupert Barrett (1903–1979) — British thoracic surgeon; eponym. 1950 distinctions on “oesophagitis” vs “peptic ulcer”; 1957 formalised the columnar-lined lower oesophagus.
- Philip Rowland Allison (1907–1974) — British surgeon; 1946/48 reflux–hernia aetiology; with Johnstone (1953) clarified organ identity and introduced the term “Barrett’s ulcer.”
- Alan Stewart Johnstone (1905–1990) — British surgeon; co-author (1953) separating reflux ulcers from gastric-type ulcers within a columnar-lined oesophagus.
- John Isaac Hayward (1910–1999) — Australian thoracic surgeon; 1961 Thorax: “the oesophagus is a tube… regardless of its lining,” foreshadowing metaplastic understanding.
- Wilder Tileston (1875-1969) — American surgeon; Early synthesis (1906) of “peptic ulcer of the oesophagus,” anchoring reflux injury at the cardia.
- Sir Morell Mackenzie (1837–1892) — English Laryngologist; 1884 clinical definition of acute oesophagitis and severe sequelae.
- Johann Friedrich Hermann Albers (1802–1865) — German Pathologist; 1833 systematic account of perforating oesophageal ulcers with aerodigestive fistulae.
- Basil Charles Morson (1921–2011) and John R. Belcher — 1952 report linking adenocarcinoma to columnar epithelium at the squamo-glandular junction.
References
Historical references
- Mackenzie M. Acute oesophagitis. In: Diseases of the throat and nose. 1884: 41-49
- Tileston W. Peptic ulcer of the oesophagus. The American Journal of the Medical Sciences. 1906; 132(2): 240–265.
- Allison PR. Peptic ulcer of the esophagus. J Thorac Surg. 1946; 15: 308-17.
- Allison PR. Peptic ulcer of the oesophagus. Thorax. 1948; 3(1): 20-42
- Barrett NR. Chronic peptic ulcer of the oesophagus and ‘oesophagitis’. Br J Surg. 1950 Oct;38(150):175-82.
- Lortat-Jacob JL. Les maladies peptiques de l’oesophage [Peptic ulcer of the esophagus]. J Int Chir. 1951 Mar-Apr;11(2):152-75.
- Allison PR, Johnstone AS. The oesophagus lined with gastric mucous membrane. Thorax. 1953; 8(2): 87-101.
- Morson BC, Belcher JR. Adenocarcinoma of the oesophagus and ectopic gastric mucosa. Br J Cancer. 1952 Jun;6(2):127-30.
- Barret NR. The lower esophagus lined by columnar epithelium. Surgery. 1957 Jun;41(6):881-94.
- Hayward J. The lower end of the oesophagus. Thorax. 1961 Mar;16(1):36-41.
- Bremner CG, Lynch VP, Ellis FH. Barrett’s esophagus: Congenital or acquired? An experimental study of esophageal mucosal regeneration in the dog. Surgery. 1970 68, 209–216. [Confirmed Metaplasia]
- Paull A, Trier JS, Dalton MD, Camp RC, Loeb P, Goyal RK. The histologic spectrum of Barrett’s esophagus. N Engl J Med. 1976 Aug 26;295(9):476-80.
- Skinner DB, Walther BC, Riddell RH, Schmidt H, Iascone C, DeMeester TR. Barrett’s esophagus. Comparison of benign and malignant cases. Ann Surg. 1983 Oct;198(4):554-65
- Reid BJ, Weinstein WM. Barrett’s esophagus and adenocarcinoma. Annu Rev Med. 1987;38:477-92.
- Spechler SJ, Zeroogian JM, Antonioli DA, Wang HH, Goyal RK. Prevalence of metaplasia at the gastro-oesophageal junction. Lancet. 1994 Dec 3;344(8936):1533-6.
- Liu W, Hahn H, Odze RD, Goyal RK. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell-containing epithelium. Am J Gastroenterol. 2009 Apr;104(4):816-24.
- Hahn HP, Blount PL, Ayub K, Das KM, Souza R, Spechler S, Odze RD. Intestinal differentiation in metaplastic, nongoblet columnar epithelium in the esophagus. Am J Surg Pathol. 2009 Jul;33(7):1006-15.
Eponymous term review
- Bani-Hani KE, Bani-Hani BK. Columnar-lined esophagus: time to drop the eponym of “Barrett”: Historical review. J Gastroenterol Hepatol. 2008 May;23(5):707-15.
- Spechler SJ, Fitzgerald RC, Prasad GA, Wang KK. History, molecular mechanisms, and endoscopic treatment of Barrett’s esophagus. Gastroenterology. 2010 Mar;138(3):854-69.
- Edgren G, Adami HO, Weiderpass E, Nyrén O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013 Oct;62(10):1406-14.
- Gindea C, Birla R, Hoara P, Caragui A, Constantinoiu S. Barrett esophagus: history, definition and etiopathogeny. J Med Life. 2014;7 Spec No. 3(Spec Iss 3):23-30.
- Pleskow DK, Erim T. Barrett’s Esophagus: Emerging Evidence for Improved Clinical Practice. 2016
- Whiteman DC, Kendall BJ. Barrett’s oesophagus: epidemiology, diagnosis and clinical management. Med J Aust. 2016 Oct 3;205(7):317-24.
- Amadi C, Gatenby P. Barrett’s oesophagus: Current controversies. World J Gastroenterol. 2017 Jul 28;23(28):5051-5067.
eponymictionary
the names behind the name
BA MA (Oxon) MBChB (Edin) FACEM FFSEM. Emergency physician, Sir Charles Gairdner Hospital. Passion for rugby; medical history; medical education; and asynchronous learning #FOAMed evangelist. Co-founder and CTO of Life in the Fast lane | On Call: Principles and Protocol 4e| Eponyms | Books |
