N-acetylcysteine
Guest Post – Dr Angela Chiew – Australian Clinical Toxicologist and Emergency Staff Specialist
Acetylcysteine is the most widely used antidote for paracetamol poisoning. Its efficacy as a specific antidote for paracetamol poisoning relies mainly on its ability to stimulate glutathione synthesis. Glutathione is essential in the metabolism of NAPQI (toxic paracetamol metabolite. Acetylcysteine is a cysteine precursor, it is hydrolysed intracellularly to cysteine, which replenishes glutathione. Acetylcysteine also supplies thiol groups, which can directly bind with NAPQI in hepatocytes and enhances non-toxic sulfate conjugation.
Traditionally acetylcysteine is given over a series of three infusions given over 20 hours. It is almost completely protective against deaths secondary to paracetamol-induced liver injury when administered within 8 hours of ingestion. The traditional three bag protocol is associated with a high rate of adverse reactions, ranging from mild to severe. These include rash, nausea and vomiting, angioedema, flushing, tachycardia, bronchospasm, hypotension and death. The most common reactions to intravenous acetylcysteine are nausea, vomiting and cutaneous systemic hypersensitivity reactions. This high rate of adverse reactions has led to increasing research and utilisation of alternative acetylcysteine regimens including the SNAP (12 h modified protocol) and the two-bag regimen (current recommended protocol in Australia and New Zealand). Both these protocols are associated with a lower rate of severe reactions.
Summary:
- N-acetylcysteine (NAC) has four possible modes of action:
- Increased glutathione availability
- Direct binding of NAPQI
- Provision of inorganic sulfate
- Reduction of NAPQI back to paracetamol
Traditional Three-Bag Regimen:
Adult dosing:
- Give 150 mg/kg NAC diluted in 200 ml of 5% dextrose IV over 15-60 minutes. Followed by:
- 50 mg/kg NAC diluted in 500 ml of 5% dextrose IV over 4 hours. Followed by:
- 100 mg /kg NAC diluted in 1000 ml of 5% dextrose IV over 16 hours.
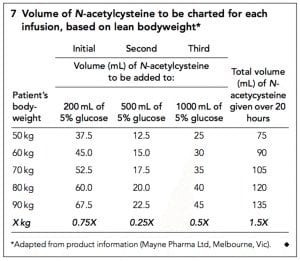
Weight based dosing table including obese patients:
Paediatric Dosing: Children < 20kg body weight:
- 150 mg/kg in 3 ml/kg of 5% dextrose over 15 minutes. Followed by:
- 50 mg/kg in 7 ml/kg of 5% dextrose over 4 hours. Followed by:
- 50 mg/kg in 7 ml/kg of 5% dextrose over 8 hours. Followed by:
- 50 mg/kg in 7 ml/kg of 5% dextrose over 8 hours.
Paediatric Dosing: Children >20kg body weight:
- 150 mg/kg in 100 ml of 5% dextrose over 15 minutes. Followed by:
- 50 mg/kg in 250 ml of 5% dextrose over 4 hours. Followed by:
- 50 mg/kg in 250 ml of 5% dextrose over 8 hours. Followed by:
- 50 mg/kg in 250 ml of 5% dextrose over 8 hours.
Two-Bag Acetylcysteine Regimen#^ – (Current Australian and New Zealand recommended protocol):
Initial Infusion:
Acetylcysteine 200 mg/kg (maximum 22 g) in glucose 5% 500 mL (child 7 mL/kg up to 500 mL) or sodium chloride 0.9% 500 mL (child 7 mL/kg up to 500 mL) intravenously, over 4 hours.^
Second acetylcysteine infusion:
Acetylcysteine 100 mg/kg (maximum 11 g) in glucose 5% 1000 mL (child 14 mL/kg up to 1000 mL) or sodium chloride 0.9% 1000 mL (child 14 mL/kg up to 1000 mL) intravenously, over 16 hours.^*
If ongoing acetylcysteine is required, continue at the rate of the second infusion (e.g. 100 mg/kg over 16 hours). Higher ongoing infusion rates (e.g. 200 mg/kg over 16 hours) may be required for massive paracetamol ingestions and a clinical toxicologist should be consulted.
- #Acetylcysteine is also compatible with 0.45% saline + 5% dextrose.
- ^For adults (age ≥ 14 years) dosing should be based on actual bodyweight rounded up to the nearest 10 kg, with a ceiling weight of 110 kg. For children (age < 14 years) use actual body weight.
- * If the initial paracetamol concentration was more than double the nomogram line following an acute ingestion increase acetylcysteine dose to 200 mg/kg (maximum 22 g) in glucose 5% 1000 mL (child 14 mL/kg up to 1000 mL) or sodium chloride 0.9% 1000 mL (child 14 mL/kg up to 1000 mL) intravenously, over 16 hours.
Note: Monitoring with pulse oximetry for the first 2 hours of the infusion is recommended.
At the completion of 20 h of acetylcysteine:
- All patients should have an ALT measured.
- For modified-release ingestions and those with an initial paracetamol concentration greater than double the nomogram line a paracetamol concentration should be repeated.
Acetylcysteine should be continued in those patients who have:
- a persistently high paracetamol concentration > 10 mg/L (66 μmol/L)
OR
- ALT is > 50 U/L and increasing.
[NOTE: small fluctuations in ALT/AST (e.g. +/- 20 U/L or +/-10%)] are common and do not on their own indicate the need for ongoing acetylcysteine).
When to increase the dose of acetylcysteine:
There is no standardised definition of “massive” paracetamol overdose. However, doses >500mg/kg or >30g are considered large. Patients with high initial paracetamol concentrations greater than double the 150mg/L at 4-hour nomogram line have been shown to have an increased risk of acute liver injury even despite early treatment with acetylcysteine. In patients with high initial paracetamol concentrations greater than DOUBLE the 150mg/L at 4-hour nomogram line it has been recommended to double the dose of the 100 mg/kg over 16 h infusion to 200mg/kg IV over 16 hours. This is the current recommendation in Australia and New Zealand but practices may vary worldwide.
In Australia and New Zealand it is currently recommended that all patients who ingest ≥ 30 g or ≥ 500 mg/kg of modified release paracetamol or have a paracetamol concentration greater than double the nomogram line should receive an increased dose of acetylcysteine.
Prolonged Acetylcysteine beyond the standard 20 h regimen:
If further doses of acetylcysteine are required beyond 20 h, e.g. for late presentations or persistently elevated paracetamol concentration or biochemical evidence of acute liver injury then repeat the 100mg/kg over 16 h infusion. For adults this is a repeat of the third bag (100 mg/kg NAC in 1000 ml of 5% dextrose IV over 16 hours) until the following criteria are met.
In those patients who require acetylcysteine beyond 20 hours. Acetylcysteine can be ceased if all the following criteria have been met:
- ALT or AST are decreasing
- INR < 2.0
- Patient clinically well
AND
For modified-release ingestions and those with an initial paracetamol concentration greater than double the nomogram line: paracetamol concentration < 10 mg/L (66 μmol/L)
Anaphylactoid reactions
The main mechanism for adverse reactions is a non-immunoglobulin (Ig) E-mediated systemic hypersensitivity (anaphylactic) reaction. This is consistent with patients suffering moderate-to-severe adverse reactions having a 2.5-fold increase in histamine concentrations without elevated tryptase concentrations. Typically, this reaction occurs after the first bag of the traditional three-bag acetylcysteine regimen. For moderate to severe reactions treat as per anaphylaxis and stop the infusion. Acetylcysteine can then be recommenced once symptoms settle at half rate for 30 minutes and then recommenced as per normal protocol.
References
- Bateman DN, Dear JW, Thanacoody HK, Thomas SH, Eddleston M, Sandilands EA, et al. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomised controlled trial. Lancet (London, England). 2014;383(9918):697-704.
- Cairney DG, Beckwith HK, Al-Hourani K, Eddleston M, Bateman DN, Dear JW. Plasma paracetamol concentration at hospital presentation has a dose-dependent relationship with liver injury despite prompt treatment with intravenous acetylcysteine. Clin Toxicol (Phila) 2016; 54(5): 405-10.
- Chiew AL, Isbister GK, Duffull SB, Buckley NA. Evidence for the changing regimens of acetylcysteine. British journal of clinical pharmacology. 2016;81(3):471-81.
- Chiew AL, Isbister GK, Kirby KA, Page CB, Chan BSH, Buckley NA. Massive paracetamol overdose: an observational study of the effect of activated charcoal and increased acetylcysteine dose (ATOM-2). Clin Toxicol (Phila). 2017;55(10):1055-65.
- Chiew AL, Isbister GK, Page CB, Kirby KA, Chan BSH, Buckley NA. Modified release paracetamol overdose: a prospective observational study (ATOM-3). Clin Toxicol (Phila). 2018;56(9):810-9.
- Chiew AL, Reith D, Pomerleau A, Wong A, Isoardi KZ, Soderstrom J, et al. Updated guidelines for the management of paracetamol poisoning in Australia and New Zealand. The Medical journal of Australia. 2020;212(4):175 – 83.
- Chiew AL, Fountain JS, Graudins A, Isbister GK, Reith D and Buckley NA. Summary statement: new guidelines for the management of paracetamol poisoning in Australia and New Zealand. MJA 2015; 203(5):215-218
- Daly FF, Fountain JS, Murray L et al. Guidelines for the management of paracetamol poisoning in Australia and New Zealand – explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres. Medical Journal of Australia 2008; 188:296-301.
- Hendrickson, R.G., What is the most appropriate dose of N-acetylcysteine after massive acetaminophen overdose? Clinical Toxicology, 2019. 57(8): p. 686-691.
- Kerr F, Dawson A, Whyte IM et al. The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Annals of Emergency Medicine 2005; 45:409-13.
- Marks DJB, Dargan PI, Archer JRH, et al. Outcomes from massive paracetamol overdose: a retrospective observational study. Br J Clin Pharmacol 2017; 83(6): 1263-72.
- Pettie, J.M., et al., Safety and Efficacy of the SNAP 12-hour Acetylcysteine Regimen for the Treatment of Paracetamol Overdose. EClinicalMedicine, 2019. 11: p. 11-17.
- Prescott LF, Illingworth RN, & Critchley JA: Intravenous N-acetylcysteine: the treatment of choice for paracetamol poisoning. British Medical Journal 1979; 2:1097.
- Wong, A. and A. Graudins, Simplification of the standard three-bag intravenous acetylcysteine regimen for paracetamol poisoning results in a lower incidence of adverse drug reactions. Clin Toxicol (Phila) 2016. 54(2): p. 115-119.
- Wong A, Isbister G, McNulty R, Isoardi K, Harris K, Chiew A, et al. Efficacy of a two bag acetylcysteine regimen to treat paracetamol overdose (2NAC study). EClinicalMedicine. 2020;20:100288.
Toxicology Library
Antidote
Dr Neil Long BMBS FACEM FRCEM FRCPC. Emergency Physician at Kelowna hospital, British Columbia. Loves the misery of alpine climbing and working in austere environments (namely tertiary trauma centres). Supporter of FOAMed, lifelong education and trying to find that elusive peak performance.
