Tetanus Vaccine
Indications and Role:
The tetanus immunization is not a live virus vaccine. A toxoid is created from the virus to form an immune reaction against the toxin produced by the bacteria. Tetanus vaccines are only given as part of combined products and are now part of most countries immunisation schedule:
- diphtheria/tetanus/acellular pertussis/inactivate polio vaccine/Haemophilus influenza type b (DTaP/IPV/Hib)
- diphtheria/tetanus/acellular pertussis/inactivated polio vaccine (DTaP/IPV or dTaP/IPV)
- tetanus/diphtheria/inactivated polio vaccine (Td/IPV)
Administration and dosing:
- Vaccines should be injected intramuscularly into the upper arm or anterolateral thigh. This is to reduce the risk of localised reactions which are more common via the subcutaneous route.
- If the patient has a bleeding disorder then a subcutaneous route is preferred.
Dosage immunisation schedule (in most circumstances, a total of five doses of vaccine should give long-term protection, WHO recommends 6):
- 1st, 2nd and 3rd dose should be given 1 month apart (usually at 2, 3 and 4 months of age as a DTaP/IPV/Hib).
- If this time frame is missed it can be initiated at any time after 2 months of age.
- If the schedule is interrupted it should be resumed and not repeated.
- The first booster should be given 3 years after completion of the primary course. Or if there has been delay in the primary course, 1 year after completion.
- The second booster should be given 10 years after the first booster. If there has been a delay in the usual regimen then the second booster may be given 5 years as a minimum interval after the first booster.
Immunisation recommendations for clean and tetanus prone wounds (UK):
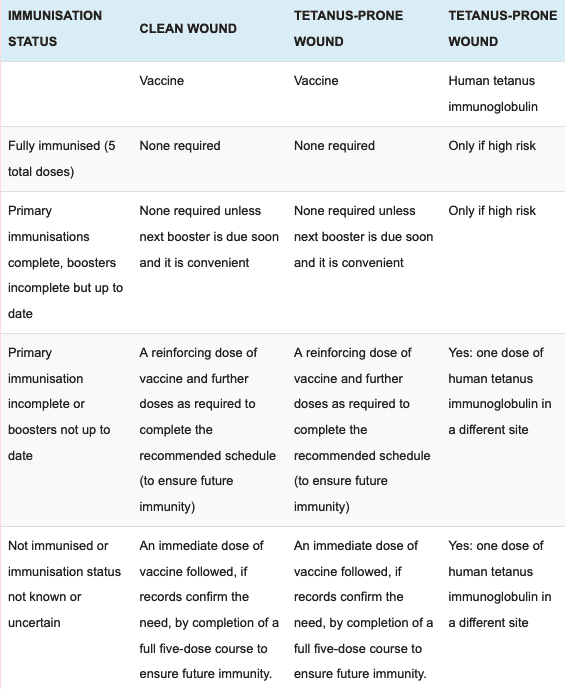
Tetanus prone wounds:
- Wounds or burns that require surgical intervention that is delayed for more than 6 hours.
- Wounds or burns that show a significant degree of devitalised tissue or a puncture-type injury, particularly where there has been contact with soil or manure.
- Wounds containing foreign bodies.
- Compound fractures.
- Wounds or burns in patients who have systemic sepsis.
High risk is regarded as any wound mentioned above that is heavily contained with material likely to contain tetanus spores and/or extensive devitalised tissue.
Contraindications or complications and special populations:
- Other vaccines can be given at the same time.
- It is safe in pregnancy and in breastfeeding mothers.
- Intravenous drug users should be given regular boosters and this should be asked regularly when they do attend a practitioner.
- HIV patients should be discussed with a specialist if they develop tetanus or have a tetanus prone wound to review their antibody response.
- Contraindications include a confirmed anaphylactic reaction to a previous tetanus containing vaccine or confirmed anaphylactic reaction to neomycin, streptomycin or polymyxin B. The US rates of anaphylaxis are 0.65 – 3 events per million doses of vaccine given.
References
Dr Neil Long BMBS FACEM FRCEM FRCPC. Emergency Physician at Kelowna hospital, British Columbia. Loves the misery of alpine climbing and working in austere environments (namely tertiary trauma centres). Supporter of FOAMed, lifelong education and trying to find that elusive peak performance.
