We need to talk about TTM…. Again
We are talking about the Targeted Temperature Management after Cardiac Arrest study (aka the TTM trial) published in the New England Journal of Medicine (Neilsen et al, 2013). This study has brought about widespread (although not universal) practice change in Australia.Whilst the conclusions of the trial were well received, there have been concerns from those involved in the trial that the study has been misinterpreted as evidence against actively cooling patients following out of hospital cardiac arrest. It is worth noting that many international bodies that set guidelines for the management of cardiac arrest have not endorsed the recommendations of the TTM trial. Currently this includes the Australian Resuscitation Council (ARC).
As has been well documented, social media played a large role in the dissemination of the findings of this study. Now that we are several months down the track I think it is worth (1) asking why these bodies have not endorsed the a target temperature of 36°C, (2) reassessing the conclusions of the TTM trial and (3) to use social media to discuss how centers have been implementing this change and what the initial experiences have been.
Why didn’t the ARC endorse a change following the TTM trial?
In December of 2013 the ARC produced a statement in response to the TTM trial. In this statement they made several observations regarding the study that are worth considering in more detail:
- Both groups were undergoing active target temperature management, which prevented fever, and was part of intensive post-resuscitation care.
- This study did not show a difference in mortality between the two target temperature groups.
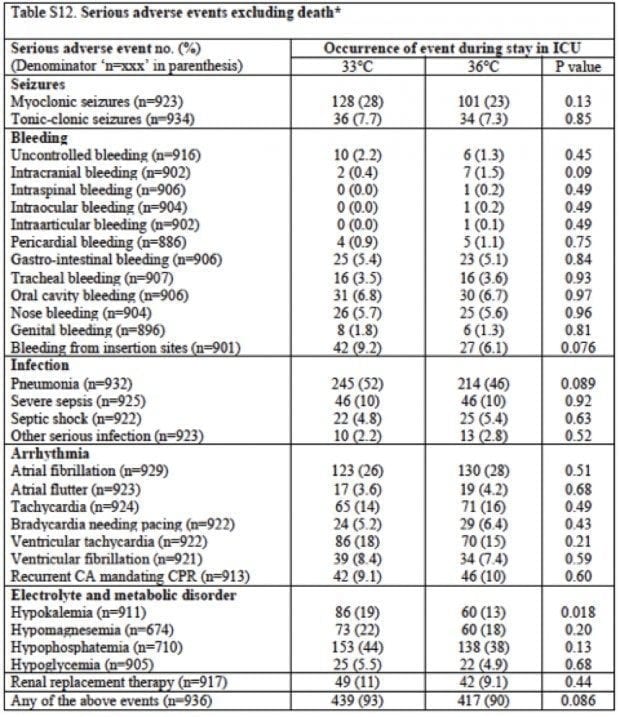
- No differences in complications between the two groups were observed.
Both groups were undergoing active target temperature management, which prevented fever, and was part of intensive post-resuscitation care.
The temperature of patients in both treatment groups was very tightly controlled as is represented below.
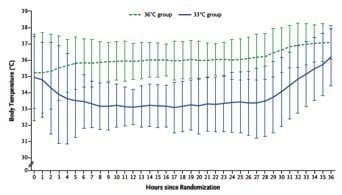
The description of how this was achieved is slightly ambiguous in the methods section of the TTM trial:
The goal was to achieve the assigned temperature as rapidly as possible with the use of ice-cold fluids, ice packs, and intravascular or surface temperature-management devices at the discretion of the sites.
More specific details of the interventions were outlined in the protocol, which was linked to the online publication. In the protocol it clearly explains:
Target temperature 36°C (TTM36): Patients will be managed at 36°C with either intravascular heat-exchange catheters or external temperature management systems. If a patient has a temperature between 30 and 33°C, the patient will be actively rewarmed to 33°C with 0.5°C/h. If a patient has an initial temperature 33-36°C, the temperature will be followed and the patient will be allowed to spontaneously rewarm and temperature management will be instituted when the core temperature is 36°C.
The fact that these patients were treated with active cooling devices seems to have been missed by many people. One of the criticisms of the TTM trial is that the authors did not report on the interventions used to control temperature in the two groups. This makes it difficult to appreciate how challenging patients in each group were to care for, and hard to adapt the study recommendations to your own practice.
Whilst it was widely reported that the TTM trial supports actively targeting normothermia, I’m concerned that there is a lack of awareness of the level of intervention that is required to achieve this in the post arrest period. Attempting to target 36C without the use of an active cooling system is not supported by the findings of the TTM trial.
This study did not show a difference in mortality between the two target temperature groups.
Prior to the TTM trial, standard practice in Australian ICUs was to target mild hypothermia of 32-34C in the post arrest period. I would argue that using cooling devices to target a temperature of 36C constitutes a novel intervention, and as such a survival benefit needed to be proved for a compelling argument to switch from standard practice. This was not the case.
In a standard superiority trial we compare a novel intervention to placebo or standard therapy. When we frame the research question we set a hypothesis (there is a difference) and a null hypothesis (there is no difference). If we cannot disprove the null-hypothesis of no difference we continue with standard therapy and do not institute the novel intervention.
The TTM trial was unusual in that the control group consisted of patients randomized to an intervention that was neither standard practice nor a placebo. As they were not able to demonstrate superiority of one group over the other, they have advocated using their control intervention.
Their justification for managing the 36C group so aggressively was a concern that allowing patients to become febrile is harmful, and thus unethical. Whilst I agree with this view, I am concerned that we are supporting use of an intervention that has (until now) only been applied to 466 patients and no study has demonstrated superiority or non-inferiority to standard practice of cooling to 32-34C.
At the time of the initial publication of the TTM trial, Simon Carley wrote a detailed analysis here on the St Emlyns blog and discussed the difference between failing to prove superiority and proving equivalence. More recently Sandra Ware wrote about a similar issue with the LINC trial of the LUCAS device, as posted here on Resus.ME.
(If you’re getting the ‘glazed over stats look’ already then skip this bit and go onto ‘No differences in complications between the two groups were observed’… Just take my word for it that they didn’t prove non-inferiority but my feeling is they probably would have done if they had designed the study to do so.)
Non-inferiority trials have been designed for situations where two treatments are likely to be very similar, but one may have other benefits over the other suh as cost, side effect profile, dosing regimen, etc. An example is the work conducted comparing dabigatran with warfarin by Connolly et al, 2009. They randomised 6,000 patients to each intervention arm and were unable to prove superiority of low dose dabigatran in stroke reduction (although the trends suggested this was probably the case) but were able to demonstrate non-inferiority.
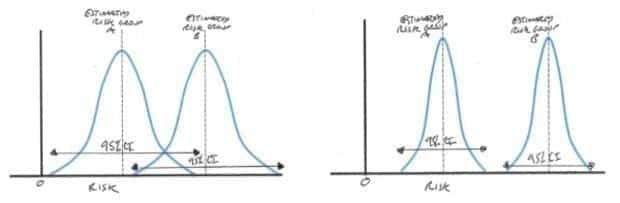
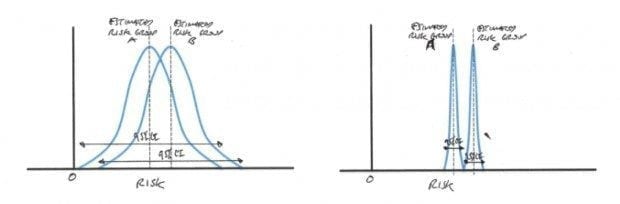
In a standard superiority trial we compare the incidence of an outcome such as mortality in the two groups and try to determine whether they are meaningfully different populations. When there is a large difference between the two groups it is relatively easy to demonstrate superiority by increasing the precision of your estimate of the population incidence with a modest increase in sample size.
When a difference between two groups is small it is difficult to prove superiority without an unrealistically large sample size resulting in a very precise estimate and narrow confidence interval (CI).
This is clear from the impact the estimated treatment effect has on sample size calculation. The problem is that unless you conduct the larger trial you cannot be certain your results are not a statistical anomaly.
Non-inferiority trials often require large sample sizes, but they are still smaller than adequately powered superiority trials of interventions with similar treatment effect or rare outcomes. In the dabigatran vs warfarin trial the incidence of CVA was 1.5% per year on warfarin. This meant that even with 6000 patients in each treatment group, superiority of dabigatran could not be proved.
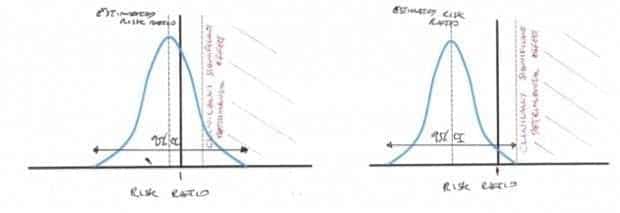
In a non-inferiority trial we estimate the treatment effect (the risk ratio is commonly used for a binary outcome such as mortality) and calculate the one sided 95% CI. A non-inferiority limit is set a priori. This is the point that a detrimental effect of the new treatment over standard practice would be considered clinically significant. Factors that affect this limit include the baseline incidence of the outcome with standard intervention and the seriousness of the outcome assessed. If the 95% CI for treatment effect does not breech this limit then non-inferiority is supported.
In the TTM trial we are looking at a primary outcome of mortality and a baseline incidence of 50% in standard therapy. The TTM data shows a risk ratio for mortality of 1.01 (favoring 36 C) with a 95% CI of 0.91 to 1.13. This means that the CI includes the possibility of an absolute risk increase for mortality of up to 5% in the 36 C group.
This result may, in fact, have been considered sufficient to prove non-inferiority, if the study had been designed to do this. However, this was not the case as a stringent non-inferiority limit with sound justification was not set a priori.
No differences in complications between the two groups were observed
One of the proposed reasons that 36 C was likely to be advantageous was the suggestion that there would be less major complications. Particular concerns regarding the use of hypothermia have been an increased risk of bleeding, incidence of dysrhythmias and the need for vasopressors and temporary pacing.
Whilst TTM was not powered to conclusively detect any minor differences, it failed to demonstrate any significant reduction in major complications. Particularly surprising was the lack of an increase in bradycardia in the T33 C group.
One of the other proposed benefits was that these patients could be cared for without the use of active cooling devices. As already mentioned, this cannot be supported by the TTM trial as both the intervention and the control arms involved active cooling.
I have heard clinicians involved in recruiting patients for TTM say that caring for patients at 36 C was actually more labour intensive than the 33 C group. There was certainly a higher incidence of shivering at 36 C (141 pts vs 156). This is not unexpected as the reason we cool to 32-34 C is that this is meant to be below the shivering threshold.
The data provided in the TTM trial only describes the number of participants who experienced shivering, but not how long, how frequent or what was required to stop it. Again this highlights the limitations of published data relating to the interventions used to maintain the target temperatures.
So why is this practice changing?
There are a lot of excellent components of TTM but I think any suggestion that this paper dictates a change in practice is an over statement. There is also the risk that confused interpretations of this paper could lead to detrimental care.
The biggest concern is the misunderstanding of the treatment the patients in the 36 C group were receiving. It is very possible that patients are receiving care that is not in concordance with the TTM protocol.
Many clinicians, including investigators involved in the TTM trial, have expressed that they believe active cooling to 33 or 36 C is now justified, and the chosen target depends on which temperature is considered easiest to manage at your centre. This is most likely correct, but the TTM trial in its published form does not conclusively prove that.
I am concerned that no secondary benefit was shown in the 36 C group and that some investigators involved in the study felt that patients are more difficult to manage at this temperature. In that case, if there is no harm in cooling to 32-34 C, why should we stop?
It is worth reading the statement made by the Australian Resuscitation Council. Their conclusion is that they still recommended 32-34C, they also state that anyone choosing to target 36C should avoid fever. I think this should include mandatory use of active cooling devices as per the TTM protocol.
The need for strong guidelines and rigorous adherence to departmental standards of care are vital during periods of practice change. Junior doctors may find themselves exposed if they decide not to initiate standard cooling without departmental agreement, and should continue to follow approved protocols for out of hospital cardiac arrest (OHCA) management.
I am interesting in hearing who is targeting 36 C, how different centers have been achieving this and what the experiences have been so far. Are active cooling devices being used routinely? How easy it has been to keep patients within their targeted parameters?
Please share your views by leaving a comment below.
References and Links
LITFL
- All in a lather over TTM
- Reports of therapeutic hypothermia’s death are greatly exaggerated
- CCC — Targeted temperature management (TTM) after cardiac arrest
Journal articles
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139-51. PMID: 19717844.
- Nielsen N, Friberg H, Gluud C, Herlitz J, Wetterslev J. Hypothermia after cardiac arrest should be further evaluated–a systematic review of randomised trials with meta-analysis and trial sequential analysis. Int J Cardiol. 2011 Sep 15;151(3):333-41. PMID: 20591514.
FOAM and web resources
- ARC — Therapeutic Hypothermia in Cardiac Arrest – An information update (2013) (pdf)
- St Emlyns — JC: What’s the target temperature for OOHCA cooling (2013)
- Resus.ME — Not finding a difference doesn’t prove equivalence (2013)

Critical Care
Compendium

We target =/36degC on arrival. We passively warm if <36degC on arrival. Shivering is frequently an issue and we use paralytic agents where necessary. Some clinicians are however, reluctant to commence paralytic infusions. After the first 24/24 we aim for normothermia/ avoid fever (37degC+/-0.5degC).