Antimicrobial Resistance: Where to from Here?
The Problem at Home and Abroad
Antimicrobial resistance (AMR) will claim 250 million lives world-wide by 2050, displacing cancer as a cause of death. The causes of AMR are multifaceted, with the lion’s share of contribution coming from various economic activities such as animal rearing, and antimicrobial manufacturing.
Whilst human health usage accounts for 20% of overall anti-microbial usage, it is a significant contributor to the problem. Clinical over-use is of most public health relevance because it selects for resistant organisms which are likely to do the most harm in hospital and other health-care settings. From both a public health perspective, over-prescription of antimicrobials selects and stabilises carriage of resistant organisms in the general population.
Antimicrobial stewardship can significantly reduce the prevalence of multi-resistant organisms (MROs) in the hospital setting. Western Australian prescribing practices were changed in the early 2000s to tackle Clostridium difficile outbreaks. These changes reduced the consumption of ceftriaxone, an extended-spectrum beta-lactam most associated with the emergence of extended-spectrum beta-lactamase- (ESBL) producing Enterobacteriaceae.
Studies of Western Australian Klebsiella pneumoniae isolates obtained over a 40-year period found that reduced ceftriaxone use coincided with the loss of ESBLs from the population. Unfortunately, newer, fitter, and more resistant Gram negative bacteria have emerged recently in WA. The establishment of these organisms in the local community has coincided with a concerning increase in antimicrobial consumption, and is promoting the development of harder-to-treat MROs.
Where to From Here?
Despite the threat posed by AMR, more people die from not receiving antibiotics. These deaths predominantly occur in developing nations where access to pharmaceuticals is limited. Excessive restriction on antimicrobial use would increase mortality in severe infections such as sepsis and meningitis, which is clearly undesirable, the immediate use of broad-spectrum agents is justified in this context.
However, in the case of non-life-threatening acute infections (e.g. uncomplicated UTI) our cognitive biases play a role in the treatment selection process. Prescribing antibiotics appears to be the least risky treatment for an individual; yet over-prescription will ultimately harm more people as common microbes become resistant. This is compounded by temporal discounting (the tendency to judge immediate events as more risky than future events) increasing the temptation to prescribe antibiotics, particularly where there is greater uncertainty surrounding the diagnosis. Removing the uncertainty surrounding these choices is therefore key to providing clinicians and patients greater confidence in withholding unnecessary treatment.
A rapid antimicrobial susceptibility test would be the optimal way to inform prescription choices at the point of care.
Rapid susceptibility tests may soon be available, with our lab’s recent development of a flow-cytometry assisted antimicrobial susceptibility test (FAST) capable of determining meropenem susceptibility within 53 minutes. Pending clinical translation, this test would enable more targeted prescription, increasing patient survival by enabling more rapid use of effective trying antimicrobials (Figure 1). At the same time this approach would also slow the emergence of increased antimicrobial resistance. While promising, it is important to keep in mind that antimicrobial susceptibility tests correlate with patient outcomes at a population level and may vary for individual patients.
Variability in patient outcomes may be the result of altered pharmacokinetic/ pharmacodynamic (PK PD) properties, and can be addressed biochemical monitoring serum antimicrobial levels (availability varies by region). These tests are of greatest utility in patients with altered PK PD profiles, e.g. those with renal disorders, and neutropenia. Combining rapid tests with PK PD profiling would result in better identification of effective antimicrobials and proper dosing, reducing patient mortality and the emergence of resistance resulting from the administration of sub-therapeutic antimicrobial doses. Ultimately, these tests must align with the clinical workflow and address issues at the coal face; making feedback from doctors and nurses essential.
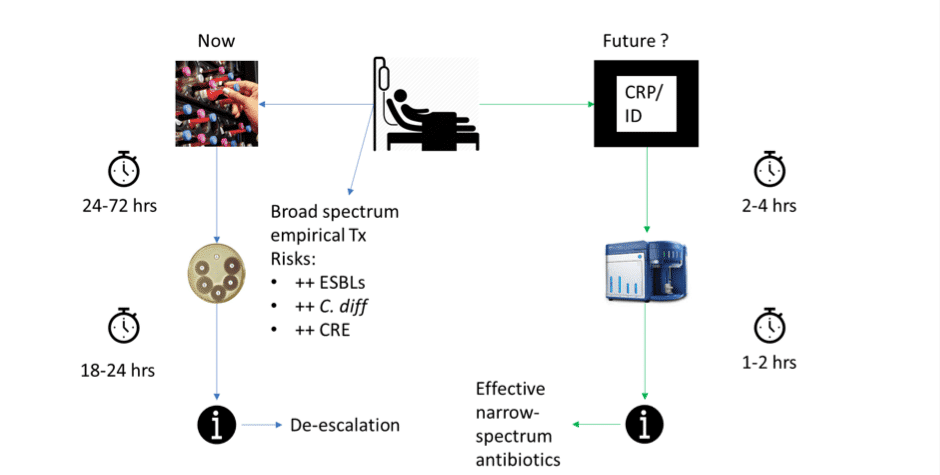
Figure 1: Comparison of current and future sepsis workflows. Conventional workflow pipeline (Now) is associated with the use of inappropriate broad-spectrum antimicrobials, increasing a patient’s risk of MRO infections. If rapid bacterial detection and ID methods are developed (Future) a translated version of our FAST system provide susceptibility results within 3-6 hours, allowing selection of the most appropriate and effective antimicrobial chemotherapy.
Conclusion
Newer tools capable of informing these early decisions are under development, but integrating an awareness of AMR into both hospital and GP practice is a key component of winning the fight against superbugs. Throughout the development process, discussion between clinicians and researchers will ensure that diagnostic tools are effective, and also meet the needs of frontline staff.
In the mean-time, cultivating an AMR aware mind-set is the best defence against over-prescription. Understanding and accepting the systematic, ubiquitous biases which affect our judgement of risk is particularly helpful. For example, doctors are expected to protect both individual and public health; roles which sometimes conflict when prescribing antibiotics. Developing the tendency to take pause and consider whether antibiotics are necessary is a simple yet powerful action which can reduce the chances of over-prescription in both the GP clinic and hospital settings.
Acknowledgements:
Guest co-author Joanna Tedeschi is a psychology student with a bachelor degree in Pathology and Lab Medicine and some experience in cancer research. Being fascinated with cognitive psychology, she is motivated to spread a general knowledge among health workers about when and how cognitive biases impact judgements of risk and decision-making. She believes that these insights can be used to improve patient outcomes and system efficiency.
References:
- Mulroney, KT, Hall, JM, Huang, X, Turnbull, E, Bzdyl, NM, Chakera, A, Naseer, U, Corea, EM, Ellington, MJ, Hopkins, KL, Wester, AL, Ekelund, O, Woodford, N, & Inglis, TJJ 2017, ‘Rapid susceptibility profiling of carbapenem-resistant Klebsiella pneumoniae’, Scientific Reports, vol. 7, Nature.
- Hall, JM, Ingram, PR, O’Reilly, LC & Inglis, TJJ 2016, ‘Temporal Flux in Beta-Lactam Resistance among Klebsiella pneumoniae in Western Australia.’, Journal of Medical Microbiology, vol. 65, no. 5, pp. 429–437.
- O’Neill, J 2014, Antimicrobial resistance: tackling a crisis for the health and wealth of nations, The Review on Antimicrobial Resistance.
- Redelmeier, D, & Tversky, A 1990, ‘Discrepancy between Medical Decisions for Individual Patients and for Groups.’ New England Journal of Medicine, vol. 322, no. 16, pp. 1162-1164. http://dx.doi.org/10.1056/nejm199004193221620.
- Sime, FB, Roberts, MS, Tiong, IS, Gardner, JH, Lehman, S, Peake, SL, Hahn, U, Warner, MS & Roberts, JA 2015, ‘Can therapeutic drug monitoring optimize exposure to piperacillin in febrile neutropenic patients with haematological malignancies? A randomized controlled trial’, The Journal of Antimicrobial Chemotherapy, vol. 70, no. 8, pp. dkv123–2375.
- Thomas, C, Stevenson, M, Williamson, DJ & Riley, TV 2002, ‘Clostridium difficile-Associated Diarrhea: Epidemiological data from Western Australia associated with a modified antibiotic policy’, Clinical Infectious Diseases, vol. 35, no. 12, pp. 1457–1462.
Last update: [last-modified]
Molecular microbiologist, Post-Doc in infectious diseases research . Research focus on ESBL and carbapenemases. Current UWA Medical student | @CdrHBiscuitIII | LinkedIn |