Exercise-associated Hyponatremia
aka Metabolic Muddle 011
A 30 year-old female was BIBA following a seizure. She was running a marathon in hot weather. Nearing the end, after 5 hours running, she was seen to fall to the ground and had a generalised tonic-clonic seizure. On arrival in the ED she was confused and agitated. Her oral temperature is 36.6 C, her chest is clear with SaO2 98% on air, she appears to have bitten her tongue and she has oedematous ankles.
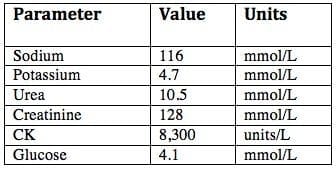
The following blood test results are available:
Questions
Q1. What is the diagnosis and what complications are present?
Answer and interpretation
Exercise-associated hyponatremia (EAH) complicated by encephalopathy (confusion and seizure). There is also evidence of seizure-induced or exercise-induced rhabdomyolysis and mild renal impairment.
EAH is defined as a blood, plasma or serum sodium level below the normal reference range (135 mmol/L) occurring within 24 hours of in an individual engaging in physical activity. Hyponatraemia is rarely present in participants at the start of endurance races (~0.8%), so EAH seems to largely develop during or after exercise. (Huw-Butler et al, 2015)
Q2. What are the risk factors for this condition?
Answer and interpretation
Risk factors for EAH include:
- maintaining or gaining weight during activities where weight loss represents euhydration
- low body weight
- prolonged exercise (typically >4 hours)
- slow speed of running associated with ease of drinking (e.g. military training exercises, marathons, triathlons, ultramarathons)
- access to fluids
- cystic fibrosis
- NSAID use (conflicting data)
- female sex (not statistically significant when adjusted for BMI and racing time)
Symptomatic EAH is becoming increasingly prevalent in non-endurance sports and has been described after relatively brief physical activity, including a Bikram Yoga session! (Huw-Butler et al, 2015)
Q3. What is the cause?
Answer and interpretation
EAH is primarily caused by
- excessive intake of hypotonic fluid during prolonged exercise, resulting in dilutional hyponatremia with an increase in total body water, and
- inadequate antidiuretic hormone (ADH) suppression preventing water clearance (due to factors such as exertion, nausea, vomiting, exertional hypovolemia, hypoglycaemia, pain and thermal stress)
Hypovolaemic EAH, with sodium loss in excess of water loss, is likely a rare occurrence (e.g. ultramarathon runners in hot environments) but may be associated with weight loss rather than weight gain. The clinical significance of this for of EAH is uncertain.
Q4. Describe the spectrum of clinical manifestations of this condition.
Answer and interpretation
Exercise-associated hyponatremia may be:
- asymptomatic
- mildly symptomatic, e.g. bloating, weakness, dizziness, headache, nausea, puffiness and weight gain (non-specific symptoms)
- severely symptomatic, e.g. EAH encephalopathy (headache, vomiting, confusion, seizures, coma) and/or acute non-cardiogenic pulmonary oedema
Symptoms depend on the magnitude of the hyponatraemia, and the rate at which sodium falls. Symptomatic EAH typically occurs with a 7 to 10% fall over 24 hours. A rapid fall to 125 mmol/L may cause more symptoms than a prolonged fall to 115 mmol/L. Asymptomatic EAH is usually detected in athletes taking part in research or undergoing blood tests for other reasons, and can is very common in some subgroups (e.g. 70% of elite rowers during a 4 week training camp and one-third of premier UK rugby league players post-match). (Huw-Butler et al, 2015)
Symptomatic EAH is uncommon, but potentially fatal (e.g. coning due to cerebral oedema).
Q5. How would you manage this patient?
Answer and interpretation
A reasonable management plan would include:
- Resuscitation — attend to ABCDEFG (don’t ever forget glucose) and vital signs.
- Secondary survey to check for evidence of injury secondary to fall/ seizure.
- Supportive treatment and monitoring.
- Fluid restriction to correct dilutional hyponatremia, but ensure adequate urine output to avoid renal failure secondary to rhabdomyolysis. Be aware that unabsorbed hypotonic fluids in the GI tract can lead to further dilutional hyponatremia. Restrict hypotonic and isotonic oral or IV fluids until urinating freely.
- Consider hypertonic saline (see Q6).
- Referral to HDU/ ICU level care for ongoing monitoring in severe cases such as this.
Q6. Is the administration of hypertonic saline indicated? What are the indications?
Answer and interpretation
Yes.
Most authorities consider the following as indications for hypertonic saline in the treatment of exercise-associated hyponatremia:
- severe hyponatremia (Na <120 mmol/L)
- significantly symptomatic (e.g. vomiting, encephalopathy or acute pulmonary oedema)
Initially, 3% hypertonic saline can be given as 100 mL boluses, twice, 10 minutes apart
- Comparable amounts of more concentrated Na+-containing solutions (eg, 10 mL of 20% NaCl; 50 mL of 8.4% NaHCO3) may be used as an alternative
- larger, more rapid boluses may be appropriate with severe EAH encephalopathy (e.g. seizures, “coning”)
- The infusion rate can be decreased following significant water diuresis
- Infusion should be stopped when the patient is asymptomatic with a normal level of consciousness
- Serum electrolytes need to be closely monitored (e.g. hourly initially)
There are no reports of cerebral pontine myelinoysis resulting from over-vigorous treatment of exercise-associated hyponatremia (an acute process) with hypertonic saline.
Oral hypertonic saline solutions (100mL 3% NaCl) can be used in milder cases of EAH.
Q7. How can this condition be prevented?
Answer and interpretation
Don’t drink too much!
Athletes should drink only according to thirst (generally no more than 400 to 800 mL/h)
Drinking to thirst is safe for endurance athletes and does not impair performance.
Some authorities recommend estimating sweat loss by serial weight measurements during prolonged exercise, but this is impractical in most situations. There is no good evidence for adding salt to fluids or for commercial sport drinks (which are generally hypotonic).
References
- Hew-Butler T et al. Statement of the Third International Exercise-Associated Hyponatremia Consensus Development Conference, Carlsbad, California, 2015. Clin J Sport Med. 2015 Jul;25(4):303-20. PMID: 26102445. [Free Full Text]
- O’Connor RE. Exercise-induced hyponatremia: causes, risks, prevention, and management. Cleve Clin J Med. 2006 Sep;73 Suppl 3:S13-8. Review. PubMed PMID: 16970148.
- Rogers IR, Hew-Butler T. Exercise-associated hyponatremia: overzealous fluid consumption. Wilderness Environ Med. 2009 Summer;20(2):139-43. PMID: 19594207
- Rosner MH, Kirven J. Exercise-associated hyponatremia. 2007 Jan;2(1):151-61. PMID:17699400
- Rothwell SP, Rosengren DJ. Severe exercise-associated hyponatremia on the Kokoda Trail, Papua New Guinea. Wilderness Environ Med. 2008 Spring;19(1):42-4. PMID 18333641
- Rothwell SP. Kokoda Medicine. LITFL
- CCC – Hypernatraemia
- CCC – Hypernatraemia Mind map (PDF)
- CCC – Hyponatraemia
- CCC – Hyponatraemia Mind map (PDF)
- CCC – Hyponatraemia Interpretation (PNG)
- CCC – SIADH – SIADH DDx
- CCC – Diabetes Insipidus Central – Diabetes Insipidus DDx
- Case – Exercise-associated Hyponatremia
- Case – Seizures, hyponatremia and ADH
CLINICAL CASES
Metabolic Muddle
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC