Fluid Responsiveness
Reviewed and revised 16 December 2015
OVERVIEW
Fluid responsiveness is variably described an increase of stroke volume or cardiac output of 10-15% after the patient receives 500 ml of crystalloid over 10-15 minutes
- Fluid responsiveness is also known as ‘volume responsiveness’ or ‘preload responsiveness’
- The definitive test for fluid responsiveness is a Fluid challenge, however this contributes to fluid overload and not all patients are ‘fluid responders’ (e.g. only about 50% of patients with shock)
- Excessive fluid administration must be avoided where possible as it leads to tissue and pulmonary oedema with consequent issues such as respiratory dysfunction, impaired would healing, intrabdominal hypertension, and poor patient outcomes
- The presumption is that increased cardiac output will lead to increased oxygen delivery (DO2) and increased tissue oxygenation — but this is not always the case, and even if it does may not improve the patient’s outcome.
Other haemodynamic parameters may be used as surrogates for stroke volume and cardiac output, with varying accuracy and reliability
PREDICTING FLUID RESPONSIVENESS
Prediction of fluid responsiveness may involve static tests or dynamic tests
- Static tests of fluid responsivenss are generally less sensitive, less specific and less useful that dynamic tests
- Use of dynamic manoeuvres to assess fluid responsiveness is an example of “functional haemodynamic monitoring”, which assesses physiological reserve by observing the response to a physiological stressor (Pinsky, 2015)
Static tests
- Clinical static endpoints (e.g. heart rate, blood pressure, collapsed veins, capillary refill time, previous urine output)
- not sensitive
- poor inter-observer reliability
- CVP/PCWP
- poor predictors
- provide a surrogate for preload only, not “preload responsiveness”
- CXR
- look for pulmonary edema
- unreliable and late marker of fluid excess
- PiCCO
- EVLW and ITBV
- ‘one off’ lactate or SvO2 (not useful)
Dynamic tests
- Passive leg raising (PLR)
- see Passive leg raise
- Technique:
- Sit patient at 45 degrees head up semi-recumbent position by adjusting the bed.
- Lower patient’s upper body to horizontal and passively raise legs at 45 degrees up by adjusting the bed.
- Maximal effect occurs at 30-90 seconds, and should then return to baseline
- can use with pulse pressure change, PPV, VTI (echo), NICCOM, carotid Doppler flow, or ETCO2 (if ventilation and metabolic status constant)
- Limitations
- Unreliable in hypovolaemia and intrabdominal hypertension
- Need to pause other interventions during the test.
- Positional changes may be contra-indicated in some patients (e.g., raised intracranial pressure)
- End-expiratory occlusion test (EEOT)
- Occluding the circuit at end-expiration prevents the cyclic effect of inspiration to reduce left cardiac preload and acts like a fluid challenge
- Technique:
- A 15 second expiratory occlusion is performed (e.g. expiratory hold on the ventilator) and an increase in pulse pressure or cardiac index predicts fluid responsiveness with a high degree of accuracy
- Response only lasts seconds
- Limitations
- The patient must be able to tolerate the 15 second interruption to ventilation without initiating a spontaneous breath
- Requires precise and real-time CO measurement
- Can only be used in mechanically ventilated patients
- Less sensitive if poor lung compliance (e.g., ARDS)
- Unreliable if spontaneous breathing, proning, or intra-abdominal hypertension
- Tidal volume challenge (TVC)
- Technique:
- Perform controlled ventilation with tidal volume >8 mL/kg PBW
- Limitations
- Can only be used in mechanically ventilated patients.
- Less sensitive if poor lung compliance (e.g., ARDS)
- Unreliable if spontaneous breathing, or intra-abdominal hypertension
- Technique:
Ultrasound (can be used dynamically)
- Echocardiography
- left ventricular outflow tract (LVOT) velocity time index (VTI) allows measurement of stroke volume
- EDV approximates preload
- Lung ultrasound
- can be used to detect pulmonary oedema, i.e. lack of fluid tolerance
- IVC ultrasound (see below)
- VEXUS grading to assess venous congestion
- evaluation of IVC diameter and venous Doppler waveform of the portal, hepatic and interlobular renal veins
- Carotid ultrasound
- carotid VTI less accurate than LVOT VTI as angle of insonance (or incidence) of the ultrasound beam to blood flow is more critical with smaller vessels
- more accessible than echocardiography in many patients
- carotid corrected flow time also being studied
Respiratory variation tests (can be used dynamically)
- IVC ultrasound
- assess size and degree of inspiratory collapse
- correlates with CVP, but CVP is a poor indicator of fluid responsiveness
- systolic pressure, pulse pressure (PPV) and stroke volume (SVV)
- see Systolic Pressure Variation
- generally limited to mechanically ventilated patients in sinus rhythm
- aortic blood velocity
SIGNIFICANCE
Fluid responsiveness does not mean that a patient should be given fluids!
- If a patient has low cardiac output that requires correction, fluid responsiveness means that stroke volume (and usually cardiac output, unless heart rate decreases) will improve if fluids are given
- It means patients are on the ascending portion of their Starling curve, in other words, they have ‘preload reserve’
- However, administered fluid may not stay in the circulation for very long and contribute to tissue and lung oedema.
- Similar cardiac responses may occur with agents such as noradrenaline that may improve preload by decreasing venous capacitance through venoconstriction.
We should probably use different cutoff values for fluid responsiveness depending on the clinical context. For example, patients with severe respiratory failure need higher specificity and lower sensitivity tests of fluid responsiveness, whereas the opposite may be appropriate in patients with pre-renal failure
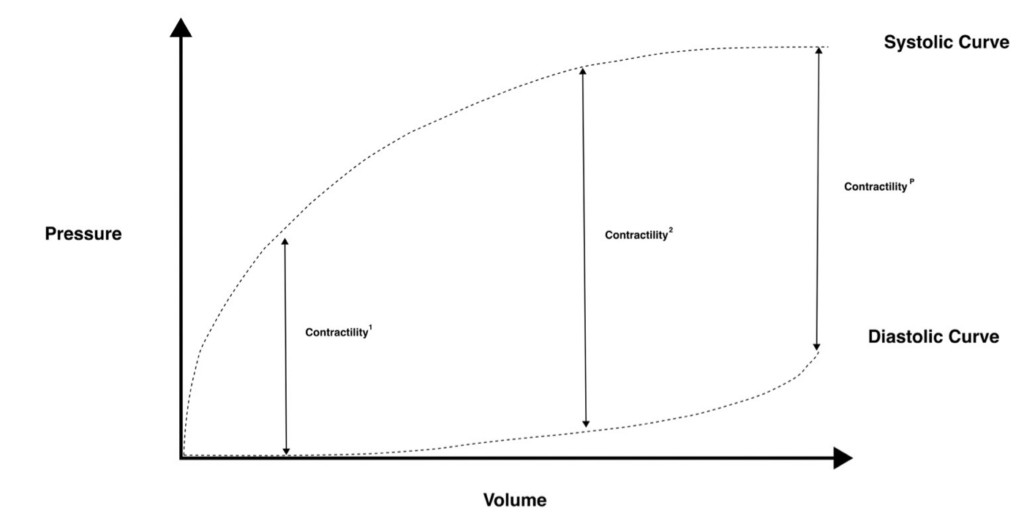
Figure. Preload responsiveness and the Frank-Starling Relationship. Note that contractility, the difference between systolic ventricular pressure and diastolic ventricular pressure, is greater at a higher EDV, which corresponds to preload. However, this no longer occurs at pathologically high EDVs (far right of the curve), where contractility fails to increment and may even decrease. “Fluid responders” are on the “steep part” of the Frank-Starling curve. Image created by Kevin Pathmanathan.
VIDEO
Fluid responsiveness by CritIQ:
References and Links
LITFL
- CCC — Fluid challenge
- CCC — Passive leg raise
- CCC — Systolic Pressure Variation
- SMACC: The Dark Art of IVC Ultrasound by Justin Bowra (2013)
Journal articles
- Cannesson M, Le Manach Y, Hofer CK, Goarin JP, Lehot JJ, Vallet B, Tavernier B. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a “gray zone” approach. Anesthesiology. 2011 Aug;115(2):231-41. PMID: 21705869. [Free Full Text]
- Durairaj L, Schmidt GA. Fluid therapy in resuscitated sepsis: less is more. Chest. 2008 Jan;133(1):252-63. doi: 10.1378/chest.07-1496. Review. PubMed PMID: 18187750.
- Horejsek J, Kunstyr J, Michalek P, Porizka M. Novel Methods for Predicting Fluid Responsiveness in Critically Ill Patients-A Narrative Review. Diagnostics (Basel). Feb 16 2022;12(2)doi:10.3390/diagnostics12020513
- Levitov A, Marik PE. Echocardiographic assessment of preload responsiveness in critically ill patients. Cardiol Res Pract. 2012;2012:819696.PMC3171766.
- Mandeville JC, Colebourn CL. Can transthoracic echocardiography be used to predict fluid responsiveness in the critically ill patient? A systematic review. Crit Care Res Pract. 2012;2012:513480. PMC3286892.
- Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009 Sep;37(9):2642-7. PMID: 19602972.
- Marik PE, Lemson J. Fluid responsiveness: an evolution of our understanding. Br J Anaesth. 2014 Apr;112(4):617-20. PMID: 24535603. [Free Full Text]
- Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011 Mar 21;1(1):1. PMC3159904.
- Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. Jun 2002;121(6):2000-8. doi:10.1378/chest.121.6.2000
- Monnet X, Teboul JL. Assessment of volume responsiveness during mechanical ventilation: recent advances. Crit Care. 2013 Mar 19;17(2):217. [PMID: 23510457. [Free Full Text]
- Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006 May;34(5):1402-7. PMID: 16540963.
- Monnet X, Bleibtreu A, Ferré A, Dres M, Gharbi R, Richard C, Teboul JL. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med. 2012 Jan;40(1):152-7. PMID: 21926581.
- Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, Teboul JL. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007 Jan;35(1):64-8. PMID: 17080001.
- Pinsky MR. Functional hemodynamic monitoring. Crit Care Clin. Jan 2015;31(1):89-111. doi:10.1016/j.ccc.2014.08.005
- Préau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010 Mar;38(3):819-25. PMID: 20016380.
- Teboul JL, Monnet X. Prediction of volume responsiveness in critically ill patients with spontaneous breathing activity. Curr Opin Crit Care. 2008 Jun;14(3):334-9. PMID: 18467896.
- Vincent JL, Weil MH. Fluid challenge revisited. Crit Care Med. 2006 May;34(5):1333-7. Review. PubMed PMID: 16557164.
FOAM and web resources
- EMCrit Podcast 64 – Fluid Responsiveness with Dr. Paul Marik (2012)
- EMCrit Podcast 86 – IVC Ultrasound for Fluid Tolerance in Spontaneously Breathing Patients – EAT IT STONE (2012)
- EMCrit — The IVC for Fluid Assessment Roundup (2013)
- Resus.ME — Predicting volume responsiveness (2013)
- Resus.ME — End expiratory occlusion (2009)
- Ultrasound Podcast — Carotid VTI Passive Leg Raise for Volume Responsiveness (2013)
- Ultrasound Podcast — Carotid Flow Time for Volume Responsiveness + “What does the Chris Fox say?” (2013)
- Ultrasound Podcast — Integrated ultrasound approach to Fluid Responsiveness……Canadian Style (2013)

Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC
