Lung Transplant
OVERVIEW
- Can have one or both lungs transplanted depending on disease process
- Single lung transplants only suitable for non-infective conditions that will not go on to damage new lung, with no cardiac disease
- pulmonary hypertension is also best treated with bilateral lung transplantation (BLT)
- most patients are extubated within 48 hours post-op
- <3% of procedures are heart-lung transplants (e.g. Eisenmenger syndrome, rarely idiopathic pulmonary hypertension, coexistent severe LV dysfunction or severe CAD)
- living-donor bilobar transplantation have been largely abandoned
INDICATIONS
Top 3:
- cystic fibrosis
- emphysema/COPD
- idiopathic pulmonary fibrosis
Others:
- alpha-1-antitrypsin deficiency
- re-transplant
- primary pulmonary hypertension
- bronchiectasis
- sarcoidosis
CONTRAINDICATIONS
- evidence of severe extra-pulmonary disease (conditions like scleroderma are controversial)
- poor nutritional or rehabilitation status
- recent or current malignancy (except non-melanoma skin cancer)
- active Hep B and C with histological evidence of liver damage
- severe psychiatric illness
- absence of social support network
- repeated noncompliance with medical care
Prior pleurodesis complicates surgery but is not a CI.
Controversies:
- age >65y
- HIV
- ventilator or ECMO dependent patients
- CF colonised with Burkholderia cepacia complex (associated with severe infections post-transplant)
TIMING
- timing of transplantation is when the likelihood of survival from transplant exceeds that without
- also takes into account other factors like quality of life, patient preference and disease-specific features
STANDARD LUNG DONOR CRITERIA
- < 55 years
- ABO compatible
- clear CXR
- PaO2 >300mmHg on FiO2 1.0 and PEEP 5
- < 20 pack year smoking history
- absence of chest trauma on side of harvest
- no aspiration or sepsis
- sputum: no bacteria, fungus, WCC (gram stain) or purulence
- negative for HIV, Hep B and Hep C
- no active or recent history of malignancy
- no history of significant chronic lung disease
These criteria are stringent and lead to only 15-25% recovery rates. Less stringent criteria are used at the Alfred, with 66% recovery rates and no decline in outcomes.
PHYSIOLOGY
- lung remains denervated
- bronchial artery circulation and lymphatics regenerate after several weeks
- regulation of breathing is not lost, as it is through chest wall efferents
- cough response lost below anastomosis
- normal ABG (unless patient reliant on hypoxic drive -> respiratory acidosis)
- normal response to exercise
- normal bronchomotor tone
POST-OPERATIVE MANAGEMENT
Basic checks
- ETT – Check position & suction, catheter passes easily? Sputum/ blood?
- ICCs
- CXR – Position of lines & tubes
- Analgesia: assess
- MAP & CI: confirm targets
- PaO2/ FiO2 ratio – Check at 0, 3, 6, 12, 24, 48, 72 hrs or more if required
Haemodynamic Management
- transplanted lung very susceptible to pulmonary oedema (lymphatic disruption, SIRS response to allograft -> reimplantation response, overzealous crystalloid use)
- cautious use of volume in first 72 hours
- use colloids, blood and albumin
Alfred Algorithm
- Targets: MAP usually 65-75 mmHg, CI usually 2.2 – 2.5 l/min/m2
- If MAP high:
— high CI -> wean vasopressors, decrease fluids, give frusemide if wet lungs (P/F ratio <300)
— low CI -> rewarm to T>36C and check CVP;
if CVP <7 -> SNP, fluid challenge
if CVP >7 -> SNP or inodilator, consider TTE - If MAP low:
— high CI -> noradrenaline (titrate to MAP), fluid challenge if CVP <4 and PF ratio >300, consider TTE
— low CI -> rewarm to T>36C and repeat basic checks
if CVP <7 -> fluid challenge, consider TTE
if CVP >7 -> assess for dynamic hyperinflation (decrease RR, TV, I-time), start adrenaline, repeat CXR, fluid challenge, consider TTE/ TOE
Ventilator Management
- double lung recipient: PEEP 5-15 (helps with oxygenation and also tamponade of small blood vessels in chest)
- single lung recipient: PEEP will be directed to native lung (more compliant)
-> acute native lung hyperinflation
-> can cause cardiac tamponade (don’t use high level of PEEP)
Guided by PF ratio (Alfred algorithm)
- >300
-> wean epoprostenol/ iNO, sedation, SIMV rate
-> then change to PSV with TV >8 mL/kg
->wean to FiO2 <0.3 and PEEP 5 and extubate if hemodynamically stable and good pain control - 200-300
-> wean epoprostenol/ iNO, sedation, SIMV rate
-> then change to PSV with TV >8 mL/kg
-> wean to FiO2 <0.4 and PEEP to 5 once epoprostenol/ iNO is off - 150-200
-> repeat basic checks, decrease TV to 6 mL/kg
-> wean to FiO2 <0.6 (SaO2 >90%), increase PEEP by 2.5 to max 10 cmH20, consider epoprostenol/ iNO
-> do not wean SIMV rate or change to PSV - <150
-> repeat basic checks, decrease TV to 6 mL/kg
-> wean to FiO2 <0.6 (SaO2 >90%), consider epoprostenol/ iNO
-> do not wean SIMV rate or change to PSV
-> consider increased PEEP by 5 cmH20 (max 15 if double lung, 10 if single lung)
-> consider increase RR by 4/min (max 30 if double lung, 16 if single lung)
Chest Physiotherapy
- very important
- transplanted lung is denervated so cough reflex impaired
Patient Positioning
- should be nursed with native lung down for 6 hours -> diminish blood flow to transplant and decrease risk of pulmonary oedema
Pain management
- epidural if coags normal; can bolus and increase rate for surgical site pain
- paracetamol
- tramadol (lowers seizure threshold especially with calcineurin inhibitors)
- ketamine and/ or opiate infusions
- pregabalin
- AVOID NSAIDS
Immunosuppression
- local protocols exist
- some involve early antibody administration -> lymphocyte depletion or IL receptor antagonism
- usually on 3 agents:
- corticosteroids
- calcineurin inhibitors (tacrolimus, cyclosporine)
- azathioprine or mycophenolate
may be delayed or dose decreased if renal dysfunction
Infectious disease prophylaxis
- nosocomial infections common – HAP, lines, chest tubes
- prophylaxis using late generation cephalosporins and vancomycin
- CMV prophylaxis sometime used as well (IV ganciclovir / PO valganciclovir)
- use of anti-fungal agent is controversial – inhaled antifungals are commonly used (aerosolised amphotericin B), may be escalated to IV
INVESTIGATIONS TO DETECT COMPLICATIONS
Bedside
- spirometry
Laboratory
- infection – CMV Ag/PCR, flu swab, legionella, mycoplasma
- sputum/ BAL – MCS incl fungi, AFB, PCP and viral panel
- immune – Ig levels, immune cell function assay, trough levels (ciclosporin, tacrolimus, sirolimus)
Imaging
- Echo
- CXR
- CTPA and CT chest
Other
- Bronchoscopy -> BAL and transbronchial biopsy
COMPLICATIONS
(1) Hypotension
- often cause by volume depletion
- gentle volume resuscitation (avoid APO)
- albumin + RBC
- if related to positive pressure (e.g. dynamic hyperinflation) -> remove from mechanical ventilation
(2) Mucous plugging
- suction, physiotherapy, early mobilisation
- bronchoscopy
- voluntary coughing (no cough reflex below the anastomosis)
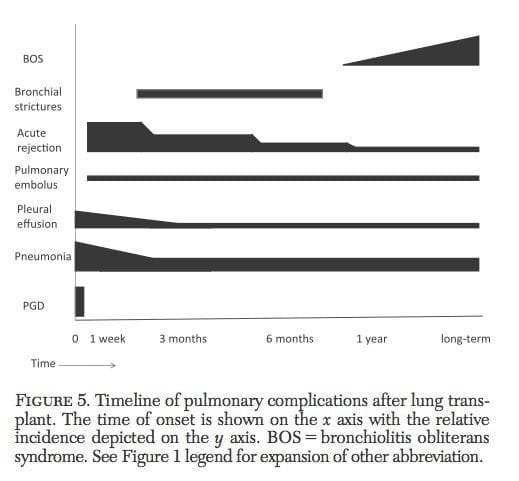
(3) Allograft problems
- implantation response aka primary graft dysfunction -> infiltrates in first few hours, may progress to ARDS (inconsistently correlates with ischemic time)
- hyperacute rejection is very rare -> retransplantation if suitable
- early rejection (acute cellular rejection) -> almost all recipients in first 3 months (can be subclinical), some very early (e.g. 48-72h) – time course distinguishes it from implantation response
- doubtful cases -> bronchoscopic washings and transbronchial biopsy -> Rx with antibiotics and pulsed steroids
- in single lung transplant -> isolate transplanted lung and ventilate native lung, native lung positioned down
- acute antibody-mediated rejection due to donor-specific anti-HLA alloantibodies may mimic early rejection and may require plasmapheresis
(4) Bleeding
- haemostatic resuscitation
- early return to OT
(5) Bronchial anastomosis problems
- dehiscence -> rare but often fatal (may be due to fungal invasion) due to PTX, mediastinitis and hemorrhage
- bronchial or tracheal stenosis may occur in the early months -> dilation or stent
- anastomosis can be inspected by bronchoscopy
(6) Pulmonary artery anastamosis problems
- PA stricture is very common -> oxygenation problems in absence of radiographic abnormalities
- diagnosis requires a pulmonary angiogram
(7) Pulmonary venous anastomosis problems
- susceptible to kinking and clot formation -> immediate and profound pulmonary oedema
- requires immediate Doppler measurement of venous anastomosis using TOE
(8) Pulmonary infection
- common – bacterial early (bronchitis > pneumonia), others = later
- prophylactic antibiotics if resident organisms
- Pseudomonas and MRSA are common bacteria
- > 6 weeks: CMV, fungi, protozoa, other viruses
(9) Complications of immunosuppressants
PROGNOSIS
- >2/3 develop broncholitis obliterans (chronic rejection)
- median survival is ~5.7 years, with 52% survival at 5 years and 29% at 10 years
- also varies by underlying condition
FUTURE DIRECTIONS
- expanded use of DCD lungs
- ex vivo reconditioning of marginal donor lungs
- improvements in immunosuppression
References and Links
Journal articles and textbooks
- Ahmad, S. Pulmonary Complications of Lung Transplantation CHEST 2011;139:402-411. PMID 21285054 [Free Full Text]
- Currey J, Pilcher DV, Davies A, Scheinkestel C, Botti M, Bailey M, Snell G. (2010) Implementation of a management guideline aimed at minimizing the severity of primary graft dysfunction after lung transplant. J Thorac Cardiovasc Surg,139(1):154-61. PMID: 19909995
- Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med. 2011 Jul 15;184(2):159-71. doi: 10.1164/rccm.201101-0134CI. Epub 2011 Mar 31. Review. PubMed PMID: 21471083. [Free Full Text]
- Kotsimbos T, Williams TJ, Anderson GP. Update on lung transplantation: programmes, patients and prospects. Eur Respir Rev. 2012 Dec 1;21(126):271-305. PMID: 23204117. [Free Full Text]
- Lung transplantation. Sixteen articles, 217 pages. Clinics in Chest Med 2011;32:199-416.
- Schuurmans M, Benden C, Inci I. Practical approach to early postoperative management of lung transplant recipients. Swiss Med Wkly. 2013 Apr 9;143:0. PMID: 23572438. [Free Full Text]
FOAM and web resources
- ICN — Lung transplants in ICU by Priya Nair (2012)
- ICN — Podcast 70: Rosenberg on Immunosuppression (2013)
- ScanCrit — ECMO as a bridge to lung transplant

Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC