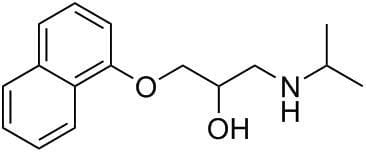
Propanolol Overdose
aka Toxicology Conundrum 044
A 27-year old female weighing 60kg presents to ED approximately one hour after swallowing 70 x 40mg propranolol tablets (= 2.8 grams) with suicidal intent. At the time of assessment she is drowsy (GCS 13) with a heart rate of 46 bpm and BP 100/60. Fifteen minutes earlier she had been awake and able to give a history to paramedics. ECG shows sinus bradycardia of 45 bpm with PR interval 210ms, QRS duration 115ms, QT interval 380ms and a small R wave in aVR of approximately 2mm in height.
Questions
Q1. What type of drug is propranolol?
Q2. Describe the toxicokinetics of propranolol.
Answer and interpretation
- Absorption: Rapidly absorbed orally. Peak blood levels occur at 1-3 hours following oral administration.
- Distribution: Highly lipophilic agent with a wide volume of distribution. Rapidly distributed to all tissues, including CNS.
- Protein Binding: Approximately 93% protein bound.
- Metabolism: Undergoes extensive hepatic metabolism, with hydroxylation of the aromatic nucleus and degradation of the isoprenaline side-chain. Over 20 metabolites identified. The 4-hydroxy metabolite has active beta-blocking properties.
- Elimination: 95-100% of an ingested dose is excreted in the urine as metabolites and their conjugates.
- Half-life: Plasma half-life is around 3-6 hours. The pharmacological effects last much longer than this. Elimination half-life is 12 hours and may be prolonged following overdose.
Q3. How does propranolol exert its toxic effects?
Answer and interpretation
Propranolol exerts its toxic effects via two main mechanisms:
- Beta-adrenergic receptor blockade (B1 and B2 receptors)
- Sodium-channel blockade (class I or “TCA-like” effect)
Competitive antagonism of beta receptors leads to decreased intracellular cAMP with blunting of the chronotropic, inotropic and metabolic effects of catecholamines.
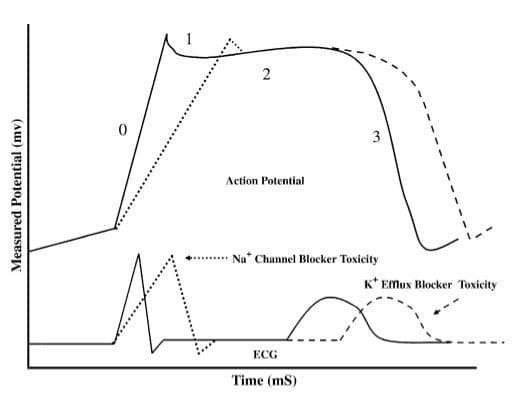
Sodium-channel blockade in the myocardium leads to prolongation of the cardiac action potential with resultant QRS widening and potential for ventricular arrhythmias.
Blockade of sodium channels in the CNS produces neurotoxic effects, i.e. seizures and coma.
Q4. What are the clinical features of propranolol overdose?
Answer and interpretation
Excess beta-adrenergic blockade causes:
- Hypotension and bradycardia
- Bradyarrhythmias, including sinus bradycardia, 1st-3rd degree heart block, junctional or ventricular bradycardia
- Bronchospasm
- Hyperkalaemia and hypo/hyperglycaemia
Sodium-channel blockade causes:
- Cardiotoxicity – QRS widening, ventricular arrhythmias and cardiac arrest
- Neurotoxicity – coma, seizures and delirium
Massive propranolol overdose typically presents with coma, seizures, bradycardia and progressive cardiogenic shock.
Q5. What are the electrocardiographic features of propranolol overdose?
Answer and interpretation
The ECG changes are similar to those seen in tricyclic antidepressant poisoning, except with a much slower ventricular rate.
Beta blockade produces:
- Sinus, junctional or ventricular bradycardia
- Prolonged PR interval
- Atrioventricular block (1st-3rd degree)
Sodium-channel blockade produces:
- Broad QRS (> 100 ms in lead II)
- Right axis deviation of the terminal QRS:
- Terminal R wave > 3 mm in aVR
- R/S ratio > 0.7 in aVR
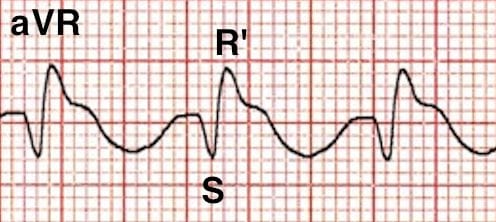
The degree of QRS broadening on the ECG is correlated with adverse events:
- QRS > 100 ms is predictive of seizures
- QRS > 160 ms is predictive of ventricular arrhythmias
An ECG showing sinus bradycardia with a 1st degree AV block and minor QRS widening (>100ms) indicates early propranolol toxicity.
Example ECG
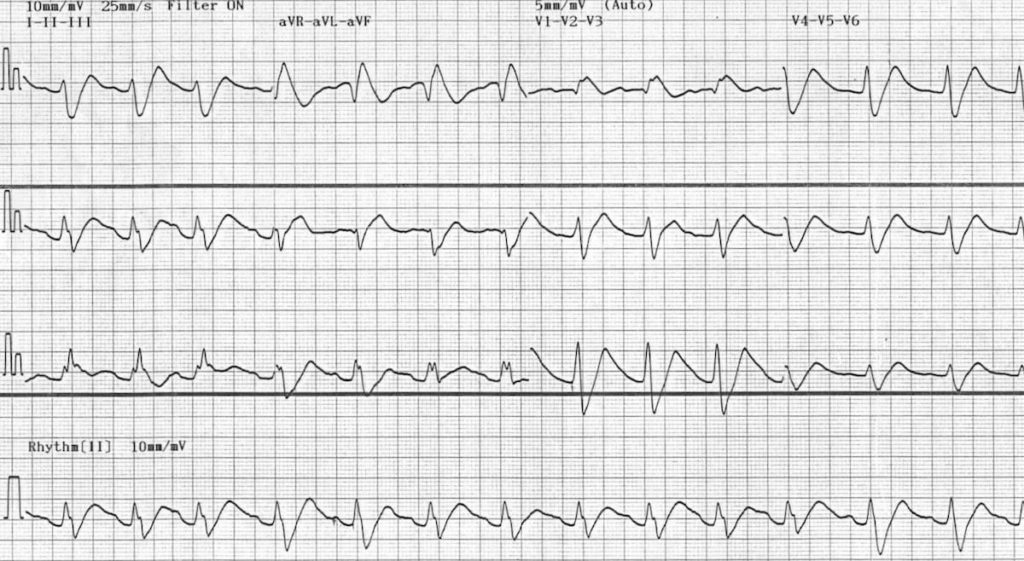
This ECG demonstrates marked sodium-channel blockade with first degree AV block and a relatively slow ventricular rate, in this case due to flecainide poisoning. Propranolol overdose would produce a very similar ECG pattern, albeit with a slower venticular rate.
Q6. What is the lethal dose of propranolol?
Answer and interpretation
- Any ingested dose of propranolol > 1 g is considered to be potentially lethal.
- Hence, this history of a 2.8 g ingestion is extremely worrying!
Q7. What is the risk assessment for this patient?
Answer and interpretation
This patient has taken a lethal dose of propranolol and is already manifesting early signs of toxicity, as evidenced by:
- Mild sedation (GCS 13)
- Bradycardia (46 bpm)
- Borderline blood pressure (100/60)
- First degree AV block (PR 210 ms)
- QRS broadening (115 ms)
Peak toxicity occurs early with propranolol (within the first 1-3 hours). This patient is likely to develop rapid onset of:
- Coma
- Seizures
- Profound hypotension and bradycardia
She will almost certainly die without aggressive medical management.
The decline in GCS over the past 15 minutes is also worrying, suggesting that this patient is going to crash and burn very soon!
Q8. How should this patient be managed?
Answer and interpretation
Propranolol overdose is managed primarily as a tricyclic antidepressant overdose (as early life-threats are due to its sodium-channel blocking effects) and secondarily as a beta-blocker overdose.
Basic Supportive Care and Monitoring
- This patient needs to be managed in a monitored area equipped for airway management and resuscitation.
- Secure IV access, adminster high flow oxygen and attach monitoring equipment.
- Treat seizures with IV benzodiazepines (e.g. diazepam 5-10mg).
- Insert an arterial line for close haemodynamic monitoring.
- Perform serial 12-lead ECGs to assess for sodium-channel blockade (QRS>100ms).
Management of Sodium Channel Blockade
- Administer IV sodium bicarbonate 100 mEq (1-2 mEq / kg).
- Intubate as soon as possible.
- Hyperventilate to maintain a pH of 7.50 – 7.55.
- If ventricular arrhythmias occur, the first step is to give moresodium bicarbonate. Lidocaine (1.5mg/kg) IV is a third-line agent (after bicarbonate and hyperventilation) once pH is > 7.5.
- In established cardiotoxicity, the dose of sodium bicarbonate can be repeated every few minutes until the BP improves and QRS complexes begin to narrow.
- Avoid Ia (procainamide) and Ic (flecainide) antiarrhythmics and amiodarone as they may worsen hypotension and conduction abnormalities.
Management of Bradycardia and Hypotension
- Treat hypotension with an initial crystalloid bolus (10-20 mL/kg). If this is unsuccessful in restoring BP, be prepared to rapidly escalate to more advanced circulatory support using inotropes and chronotropes.
- Atropine (10-30 mcg/kg) can be used as a temporising measure for bradycardia.
- Persistent bradycardia and hypotension is better treated with a titrated infusion of adrenaline or isoprenaline via a central venous catheter.
- If inotropes are required, consider early initiation of high-dose insulin euglycaemic therapy (as described in toxicology conundrum 028). This treatment appears to be very effective in massive propranolol overdose but takes time to work (30-45 minutes).
- Glucagon (once considered to be the “antidote” to beta-blocker poisoning) is no longer recommended as it is difficult to source in adequate quantities and offers no advantages over standard inotropes and chronotropes.
Screening Tests
- Paracetamol level, blood sugar and 12-lead ECG are recommended as initial screening tests in all patients with deliberate self-poisoning.
Decontamination
- Once the airway is secure, place a nasogastric tube and give 50g (1g/kg) of activated charcoal. This may reduce the dose of propranolol absorbed from the gut, limiting the duration and severity of toxicity. As propranolol is rapidly absorbed, this treatment is most likely to be effective if given early, i.e < 1-2 hours post ingestion (NB. Do not give pre-intubation due to risk of charcoal aspiration).
Disposition
- Admit the patient to the intensive care unit for ongoing monitoring and supportive care.
Q9. What do you do if the patient arrests?
Answer and interpretation
Cardiac arrest may occur in propranolol overdose due to sudden ventricular arrhythmias (i.e. pulseless VT) or progressive cardiogenic shock culminating in bradycardic PEA.
- Start CPR – Good quality CPR may be life saving. Conversely, cardioversion / defibrillation is unlikely to be successful in the poisoned heart.
- Call for help! – Toxicological arrest is approached differently to cardiac arrest from other causes. If you have not yet enlisted the advice and support of a clinical toxicologist in managing this patient, then get them on the phone now! (this is assuming that you have a spare person to talk to them… ideally get help before they arrest!)
- Push bicarb – Give boluses of IV sodium bicarbonate 1-2 mEq/kg every 1-2 minutes until return of perfusing rhythm.
- Intubate and hyperventilate (if you have not done so already).
- Give adrenaline – 1mg every 3-5 minutes as per standard cardiac arrest algorithms.
- Avoid amiodarone – It will only make things worse.
- Consider intralipid – The role of intravenous lipid emulsion (IVLE) in propranolol poisoning is not clearly defined, but it may be considered in cardiac arrest refractory to other measures. The dose is 100ml of 20% IVLE (1-1.5ml/kg) as an IV bolus over 1 minute; repeated once or twice at 3-5 minute intervals if required, followed by an infusion.
- Don’t give up! – There are numerous case reports of excellent outcomes after prolonged CPR (>45 minutes) in patients who have arrested due to poisoning.
To illustrate this last point, we had a 55-year old lady come through our department recently with a 4 gram (i.e. absolutely massive) propranolol ingestion. She had a tonic-clonic seizure in front of paramedics and promptly arrested. The paramedics continued CPR (with no drugs) for 30 minutes prior to arrival in hospital. On arrival to ED, she was treated aggressively with bicarbonate, adrenaline and high-dose insulin and achieved return of spontaneous circulation after a further 15 minutes of CPR. She was transferred to ICU on high-dose insulin (2 units/kg/hr) and adrenaline infusions, which were subsequently weaned off over the following 24-48 hours. Post-arrest echocardiogram was entirely normal (no evidence of LV dysfunction) and serial bloods showed only trivial elevations in troponin and transaminases with preserved renal function (i.e. no evidence of ischaemic ATN). She was discharged home on day 4 entirely neurologically intact!
References
- Holstege CP, Eldridge DL, Rowden AK. ECG manifestations: the poisoned patient. Emerg Med Clin North Am. 2006 Feb;24(1):159-77 [PMID 16308118]

CLINICAL CASES
Toxicology Conundrum
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC