Verapamil overdose
aka Toxicology Conundrum 028
A pharmacist in the Gibson Desert wanted to take part in the recent mass homeopathic overdose protest organized by the 10:23 movement. Unbeknown to him, the cleaning lady had been around and accidentally mixed up the homeopathic pills with slow release verapamil. After gulping down a couple of handfuls of pills, the pharmacist’s heightened gustatory awareness alerted him to the presence of verapamil in the tablets. Although he remains asymptomatic, he has a feeling that something bad might happen.

Can you answer these questions and help stop the unfortunate pharmacist from biting the dust?…
Questions
Q1. What is your risk assessment?
Answer and interpretation
All deliberate self-poisonings of verapamil are considered potentially lethal.
Serious toxicity can result from:
- >10 tablets of verapamil SR (160mg or 240mg SR capsules) or diltiazem SR (180mg, 240mg or 360mg SR capsules)
- 1-2 SR tablets of either verapamil or diltiazem in children
Q2. What are the clinical features and course of verapamil overdose?
Answer and interpretation
The onset of symptoms with standard preparations is typically within 1-2 hours of ingestion. However, with slow release preparations the onset of significant toxicity may be delayed 12-16 hours with peak effects after 24 hours.
The main clinical features relate to cardiovascular toxicity (see Q3), although metabolic disturbance is also important (see Q4).
- The early signs of toxicity are usually bradycardia, first degree heart block and hypotension. This may progress to refractory shock and death without appropriate intervention. Complications may include myocardial ischemia, stroke and non-occlusive mesenteric ischemia.
- Seizures and coma are rare and usually signify the presence of a coingestant. However, they can occur as a late feature of toxicity due to cardiovascular collapse.
Q3. How does verapamil cause cardiovascular toxicity?
Answer and interpretation
Verapamil is a calcium-channel blocker – it binds the alpha-1 subunit of L-type calcium channels, preventing the intracellular influx of calcium. These channels are functionally important in cardiac myocytes, vascular smooth muscle cells, and islet beta cells.
Verapamil: cardiac toxicity results from
- excessive negative inotropy: myocardial depression
- negative chronotropy: sinus bradycardia
- negative dromotropy: atrioventricular node blockade
Verapamil: effects on vascular smooth muscle tone result in
- decreased afterload
- systemic hypotension
- coronary vasodilation
Q4.What are the metabolic effects of overdose of calcium channel blockers like verapamil?
Answer and interpretation
The metabolic effects of calcium channel blockers like verapamil are less well known than the cardiovascular effects.
Under the stress of the drug-induced shock state, cardiac myocytes shift from using free fatty acids, their favoured “resting state” energy substrate, to carbohydrates.
But verapamil impairs this adaptive mechanism – its effects in overdose include:
- impaired uptake of glucose and free fatty acids by cardiac myocytes
- inhibition of calcium-dependent mitochondrial activity required for glucose catabolism
Furthermore, insulin release is dependent on calcium influx into islet beta cells through L-type calcium channels. This is also impaired in verapamil toxicity leading to hypoinsulinaemia which together with calcium channel blocker-induced insulin resistance results in hyperglycaemia and a ketoacidotic state.
Hyperglycemia at presentation is a recognised indicator of severe toxicity.
Impaired glucose metabolism and shock also lead to lactic acidosis.
Q5. Is there any role for decontamination in this case?
Answer and interpretation
Yes.
In the asymptomatic patient who presents early, activated charcoal can be given:
- within 1 h of ingestion for standard release preparations
- within 4 hours of ingestion for SR preparations
Whole bowel irrigation can also be performed following the administration of activated charcoal if the patient meets these criteria:
- cooperative (the pharmacist in this case will probably insist on it)
- presents within 4 hours of ingestion of >10 tablets of verapamil or diltiazem SR
- no evidence of established toxicity
However, WBI is not without risks and some experts advise against it’s use.
Q6. What specific therapeutic measures are available for treating verapamil toxicity?
Answer and interpretation
Atropine, calcium boluses and infusions, glucagon, inotropes and vasopressors, and cardiac pacing have all been advocated for managing CCB toxicity, despite questionable efficacy. Extracorporeal circulatory support and intra-aortic balloon counterpulsation have been used as heroic last ditch measures.
It is useful to have a step-wise approach to managing calcium channel blocker overdose. Early invasive blood pressure monitoring is wise in the presence of evolving hypotension and shock, as is early intubation and ventilation when life-threatening toxicity is anticipated.
The step-wise approach:
Fluid resuscitation
- up to 20 mL/kg crystalloid
Calcium
- can be a useful temporising measure to increase HR and BP
- options
- 10% calcium gluconate 60mL IV (0.6-1.0 mL/kg in children)
- 10% calcium chloride 20mL IV (0.2 mL/kg in children) [must be given via CENTRAL VENOUS ACCESS – it burns!]
- repeat boluses can be given up to 3 times
- consider calcium infusion to keep serum calcium >2.0 mEq/L
Atropine
- 0.6mg every 2 min up to 1.8 mg
Catecholamine infusions
- titrate to effect
- options include dopamine, adrenaline and/ or noradrenaline
Sodium bicarbonate
- consider in severe metabolic acidosis – 50-100 mEq sodium bicarbonate (0.5-1.0 mEq/kg in children)
Cardiac pacing
- electrical capture may be difficult to achieve and may not improve overall perfusion
- use ventricular pacing to bypass AV blockade, typical with rates not in excess of 60/min
If all else fails consider extracorporeal circulatory support or intra-aortic balloon counterpulsation – but for these to be effective you have to get the ball rolling early…
In Western Australia we tend not to use glucagon for calcium channel blocker toxicity. The experience of the clinical toxicologists at the Western Australia Poisons Information center suggests that it is not useful, and the supporting evidence for its use glucagon is limited to small, non-blinded animal studies where no survival benefit or improvement in mean arterial pressure was shown, although heart rate improved in some cases.
What about high-dose insulin euglycemic therapy? See Q7.
Q7. What is the role of HIET?
Answer and interpretation
HIET (high-dose insulin euglycemic therapy) was first used to treat verapamil toxicity in humans in 1993, with a favourable outcome. Since then, in addition to animal studies, there have been about 70 cases reporting the beneficial use of HIET in humans, with an overall survival rate of 85%. HIET has gained widespread acceptance as a core therapy for calcium channel blocker toxicity among clinical toxicologists, even though no randomised controlled trials have been performed to test its efficacy.
Unfortunately, awareness of HIET outside of toxicology circles remains poor, and there is often reluctance to administer the high doses of insulin that are recommended.
The place of HIET in the step-wise approach to managing cardiovascular toxicity has evolved. Formerly considered a last ditch measure, early is use is increasingly advocated. This is particularly important as the beneficial effects of HIET are not immediate.
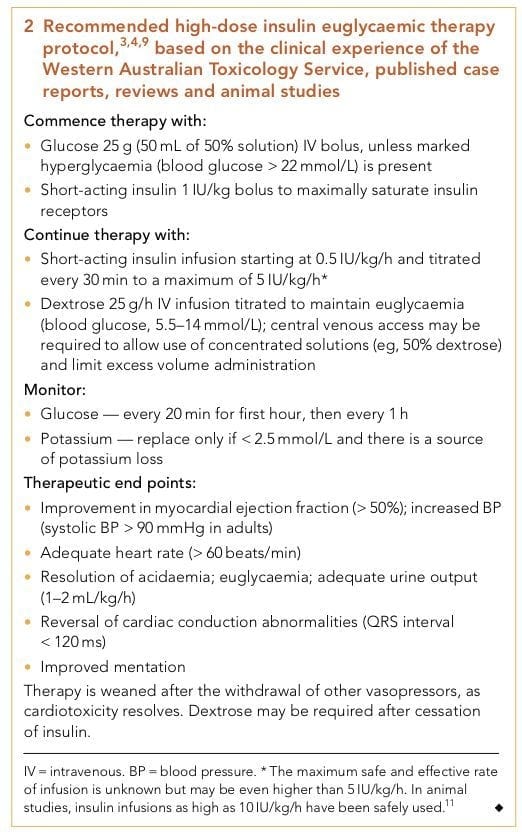
** Recommended high-dose insulin euglycemic therapy protocol based on the clinical experience of the Western Australian Toxicology Service, published case reports, reviews and animal studies [PMID 19769561] [PDF]
Q8. How does HIET work?
Answer and interpretation
HIET may allow the heart to overcome the metabolic starvation that results from calcium channel blocker toxicity (see Q4), which compounds the direct cardiotoxic effects (see Q3).
How does it do this?
The effects of insulin are numerous:
- increased glucose and lactate uptake by myocardial cells
- improved myocardial function without increased oxygen demand
- increased pyruvate dehydrogenase activity, thus hastening myocardial lactate oxidation and clear the cytosol of glycolytic byproducts that can impair calcium handling and cause diastolic dysfunction.
- promotes excitation–contraction coupling and contractility because increased glucose availability results in:
- increased sarcoplasmic reticulum-associated calcium ATPase activity
- increased cytoplasmic calcium concentrations
- enhanced calcium entrance into mitochondria and sarcolemma
HIET may be best used adjunctively with other measures such as catecholamines, for two reasons:
- insulin-mediated inotropy is not catecholamine-mediated, and is not affected by β blockers, so additive effects are likely.
- although insulin appears to improve myocardial contractility, it has no chronotropic effect and may cause vasodilation.
Q9. How safe is HIET?
Answer and interpretation
It is surprisingly safe!
Adverse events are predictable, uncommon, and easily managed.
For instance, there were NO adverse effects in these extreme examples:
- the inadvertent administration of a 1000 IU insulin loading dose for verapamil toxicity
- treatment of toxic cardiogenic shock for 2 days with a 6 IU/kg/h insulin infusion
Adverse effects of HIET include:
- hypoglycaemia (<3.3 mmol/L in about 16% of cases)
- hypokalaemia
- hypomagnesaemia
- hypophosphataemia
Additional comments:
Hypoglycemia:
- Some cases of severe calcium channel blocker toxicity in patients presenting with hyperglycaemia do not require any additional glucose administration despite high-dose insulin therapy.
- hypoglycaemia may be more likely in milder cases without marked hypotension.
Hypokalaemia (potassium < 3.5 mmol/L):
- noted in only two of seven in Greene et al’s small series, with a minimum potassium level of 2.8 mmol/L.
- Excessive correction of hypokalaemia should be avoided, because it reflects the intracellular shift of potassium from the extracellular compartment due to the action of insulin, rather than a potassium-depleted state.
- Hypokalaemia in HIET may actually be beneficial:
- it may augment myocardial contractility by enhancing calcium entry during systole
- increased intracellular potassium may have a membrane-stabilising effect in excitable cells.
References
- Bailey B (2003). Glucagon in beta-blocker and calcium channel blocker overdoses: a systematic review. Journal of toxicology. Clinical toxicology, 41 (5), 595-602 PMID: 14514004
- Greene SL, Gawarammana I, Wood DM, Jones AL, & Dargan PI (2007). Relative safety of hyperinsulinaemia/euglycaemia therapy in the management of calcium channel blocker overdose: a prospective observational study. Intensive care medicine, 33 (11), 2019-24 PMID: 17622512
- Kerns W 2nd (2007). Management of beta-adrenergic blocker and calcium channel antagonist toxicity. Emergency medicine clinics of North America, 25 (2) PMID: 17482022
- Nickson CP, Little M. Early use of high-dose insulin euglycaemic therapy for verapamil toxicity. Med J Aust. 2009 Sep 21;191(6):350-2. [PMID 19769561] [PDF]

CLINICAL CASES
Toxicology Conundrum
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC
