Tardive dyskinesia
Tardive dyskinesia is a medication-induced, chronic hyperkinetic movement disorder most commonly associated with prolonged exposure to dopamine receptor-blocking agents (DRBAs)—especially first-generation antipsychotics.
Symptoms persist for at least 1 month after cessation of the offending agent, in contrast to other extrapyramidal syndromes (EPSs) that present acutely and resolve with discontinuation.
This disorder is often permanent once established. As current therapies provide only partial symptomatic relief, early detection and prevention are critical. The most effective agents for symptom relief are VMAT2 inhibitors, though their efficacy is modest.
Tardive dyskinesia is disfiguring, disabling, and socially stigmatizing, significantly impacting quality of life.
History
- Neuroleptics were introduced in the early 1950s and transformed psychiatric care.
- By 1957, M. Schonecker, a German psychiatrist, described the first case of paroxysmal dyskinesia in a patient on megaphen (a phenothiazine).
- The term “tardive dyskinesia” was coined by Arild Faurbye in 1964, highlighting the delayed onset of symptoms.
- Valbenazine was the first FDA-approved VMAT2 inhibitor for treatment.
Epidemiology
First-generation antipsychotics:
- Annual incidence: 5–6%
- Older adults: 10–25%
- Cumulative risk: 25–50% over 5–10 years
Second-generation antipsychotics:
- Annual risk: ~4%
- Risk varies by agent:
- High: risperidone, paliperidone
- Intermediate: aripiprazole, olanzapine, ziprasidone
- Low/absent: quetiapine, clozapine
Despite second-generation use, TD prevalence remains high due to expanded antipsychotic use.
Physiology
VMAT2 (vesicular monoamine transporter‑2) packages dopamine, norepinephrine, serotonin, and histamine into presynaptic vesicles.
- VMAT1 – peripheral and central nervous system
- VMAT2 – central nervous system only
Pathophysiology
TD mechanisms likely include:
- Dopamine D2 receptor supersensitivity
- Chronic D2 blockade leads to upregulated receptor responsiveness
- Explains symptom suppression with increased dopaminergic blockade
- D1/D2 receptor imbalance
- Striatal disinhibition and unopposed D1 activity may promote hyperkinesia
- Heterogeneity of dopamine receptors
- D3, D4, and D5 receptors may also contribute
- VMAT2 inhibition leads to presynaptic dopamine depletion
Causative agents
Antipsychotics:
- First-generation (strongly associated)
- Second-generation (less strongly, but still relevant)
Antiemetics:
- Metoclopramide
- Prochlorperazine
Genetics:
- D3 receptor MscI polymorphism implicated
Bradykinesia vs hyperkinesia
- Bradykinesia: rigidity, masked face, slow movements
- Hyperkinesia: akathisia, chorea, dystonia, myoclonus, stereotypy, tremor
Clinical features
Characterised by repetitive, involuntary movements, often involving the tongue, lips, and face.
Types:
- Orofacial hyperkinesia: buccolingual stereotypy, tardive blepharospasm
- Akathisia: restlessness, pacing, subjective urge to move
- Dystonia: sustained postures (e.g., torticollis), may respond to anticholinergics
- Myoclonus / tics: jerking movements
- Parkinsonian features: controversial, pill-rolling tremor, rigidity
- Chorea: writhing movements
- Athetosis: snake-like, slow twisting movements
- Tardive tremor: high-amplitude 3–5 Hz tremor
- Ballismus: flinging movements, usually unilateral
- Other: unclassified abnormal movements
Modulating factors:
- Rest: movements present
- Voluntary movement: reduces symptoms
- Tactile suppression: may help
Assessment – AIMS
The Abnormal Involuntary Movement Scale includes:
- 7 item motor score (facial, extremities, trunk)
- 3 item global severity
- 2 item dental history
Used for monitoring and treatment efficacy, especially in clinical trials.
Diagnostic criteria (DSM-IV)
- ≥3 months cumulative DRBA exposure (or ≥1 month if age >60)
- Symptoms persist ≥1 month after cessation
- May arise during exposure or soon after withdrawal
- Must differentiate from neuroleptic-withdrawal emergent dyskinesia (resolves <4 weeks)
Investigations
Clinical diagnosis
Exclude structural causes:
- CT/MRI: normal
- PET: may show increased glucose metabolism (non-diagnostic)
Management
1. Preventive
- Avoid/cease DRBAs if possible
- If not:
- Lowest effective dose
- Avoid concurrent dopamine antagonists (e.g. metoclopramide)
- Prefer clozapine in ongoing treatment
2. Pharmacological
VMAT2 inhibitors (only approved agents):
- Valbenazine
- Deutetrabenazine
Adjunctive:
- Clonazepam – myoclonus
- Botulinum toxin – focal dystonia
3. Surgical
- Deep brain stimulation (globus pallidus): emerging role
4. Psychological support
- TD is psychosocially disabling
- Involves mental health referral and support
Disposition
Urgent referrals:
- Psychiatrist
- Psychologist
- Neurologist/movement disorder specialist
Appendix 1 – Relative propensity for EPS
| Drug | Propensity |
|---|---|
| Amisulpride | + |
| Aripiprazole | + |
| Asenapine | + to ++ |
| Brexpiprazole | + |
| Chlorpromazine | ++ |
| Clozapine | – |
| Flupentixol | ++ to +++ |
| Haloperidol | +++ |
| Lurasidone | + to ++ |
| Olanzapine | – to + |
| Paliperidone | + |
| Periciazine | + |
| Quetiapine | – to + |
| Risperidone | + |
| Ziprasidone | + |
| Zuclopenthixol | += to +++ |
Legend: – = negligible | + = infrequent | ++ = moderate | +++ = frequent
Appendix 2 – Overlap of movement disorders
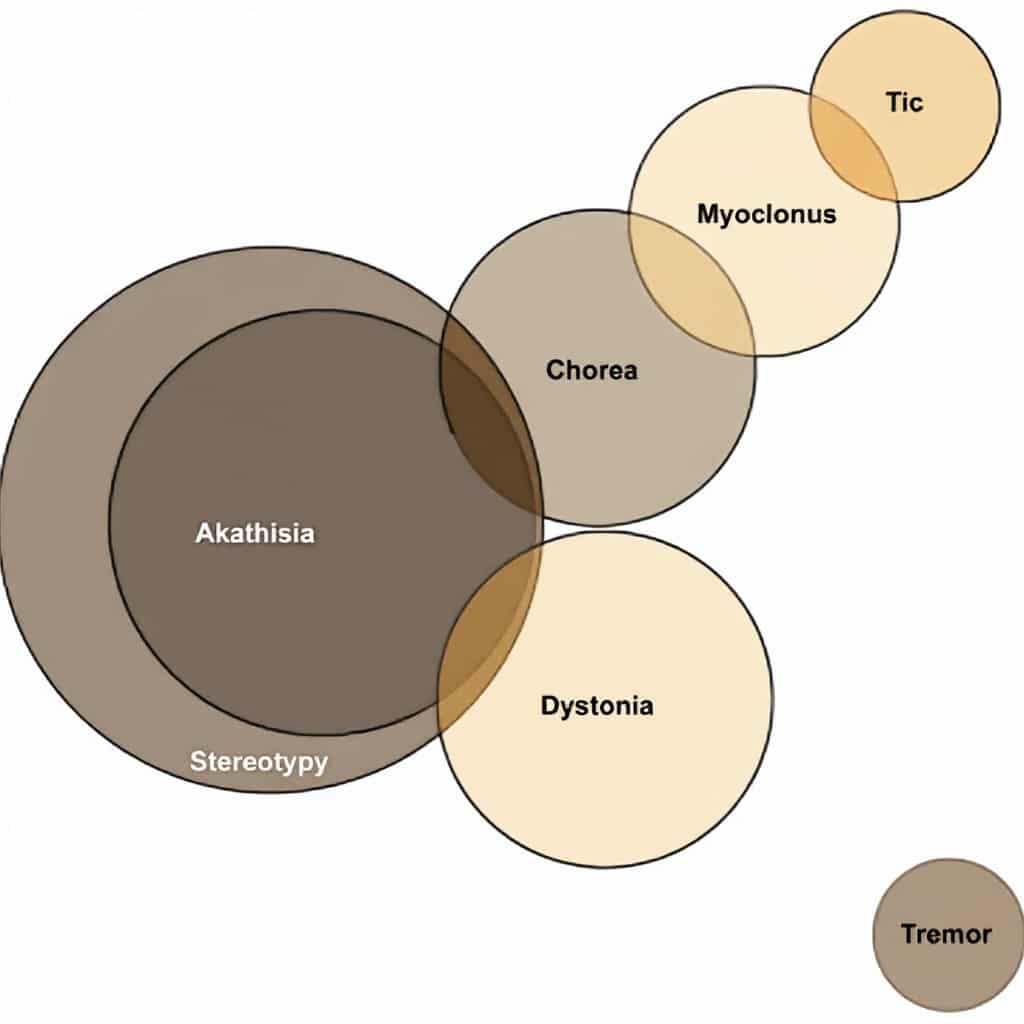
From the Venn diagram it can be seen that every case of akathisia is also a case of stereotypy. The presence of akathisia therefore implies the presence of stereotypy.
- Some cases of chorea can be classified as akathisia and stereotypy, whereas other cases of chorea can be classified as myoclonus.
- Some cases of tics can be classified as myoclonus.
- Some cases of dystonia can be classified as akathisia.
- Tics are entirely separate and distinct from akathisia, chorea, dystonia, stereotypy, and tremor.
- Tremor is distinct from the other movement disorders.
Appendix 3 – Mechanism of action of the VMAT2 inhibitors
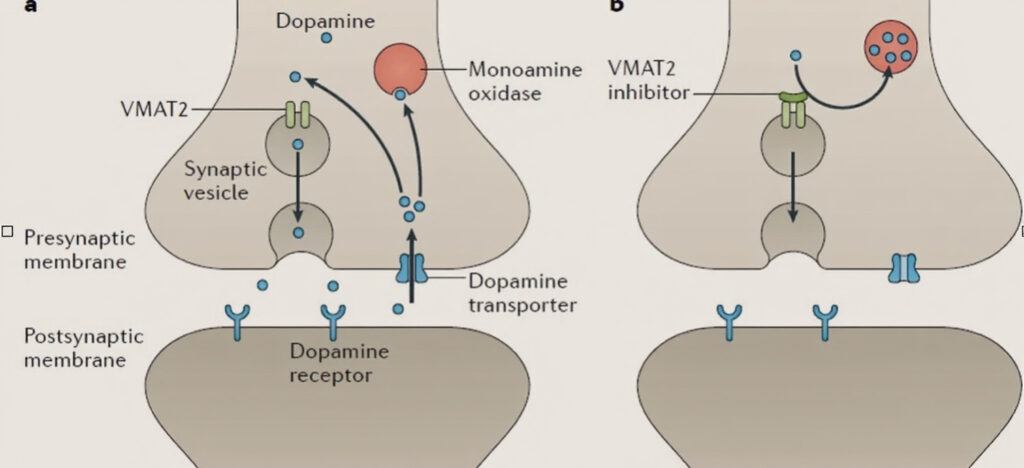
- A: Normally, vesicular membrane transport type 2 (VMAT2) mediates loading of dopamine into synaptic vesicles for release. Breakdown of dopamine is mediated by monoamine oxidase.
- B: VMAT2 inhibitors block transport of dopamine into synaptic vesicles, reducing dopamine release and depleting dopamine levels through its breakdown by monoamine oxidase.
Appendix 4 – Proposed mechanism of action of the VMAT-2 Inhibitors (Stahl 2017)
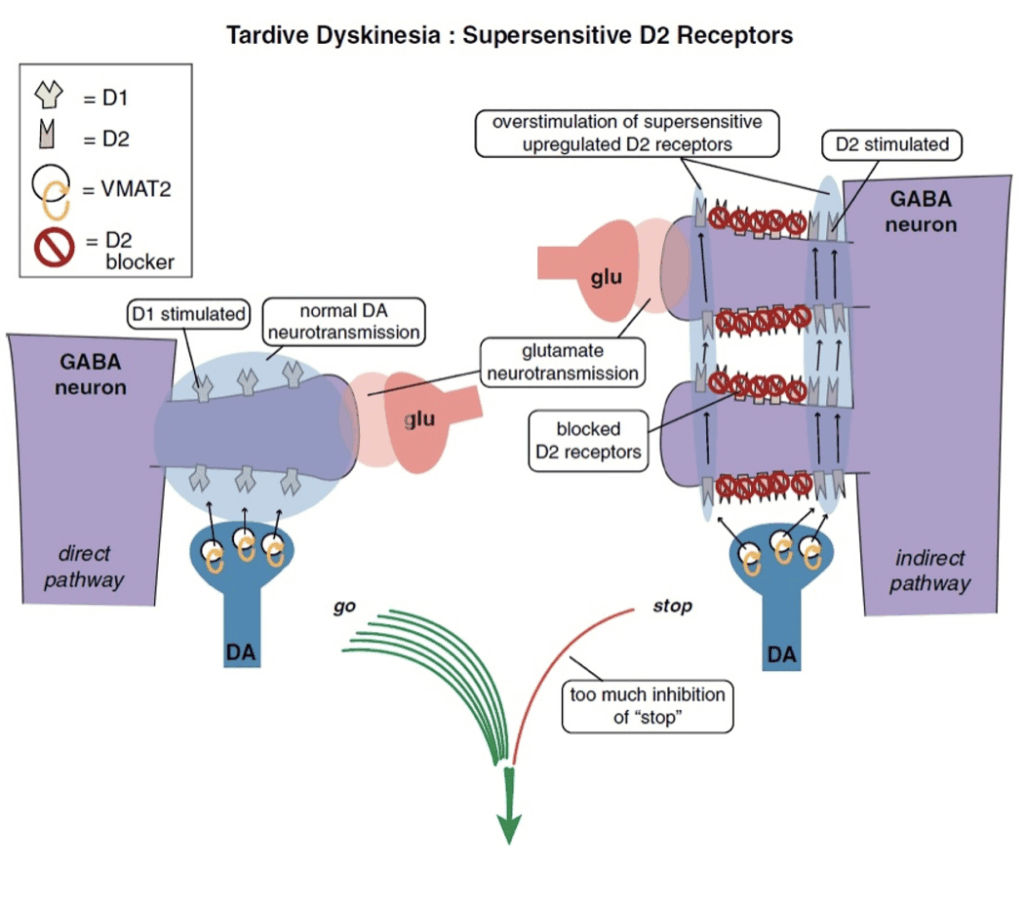
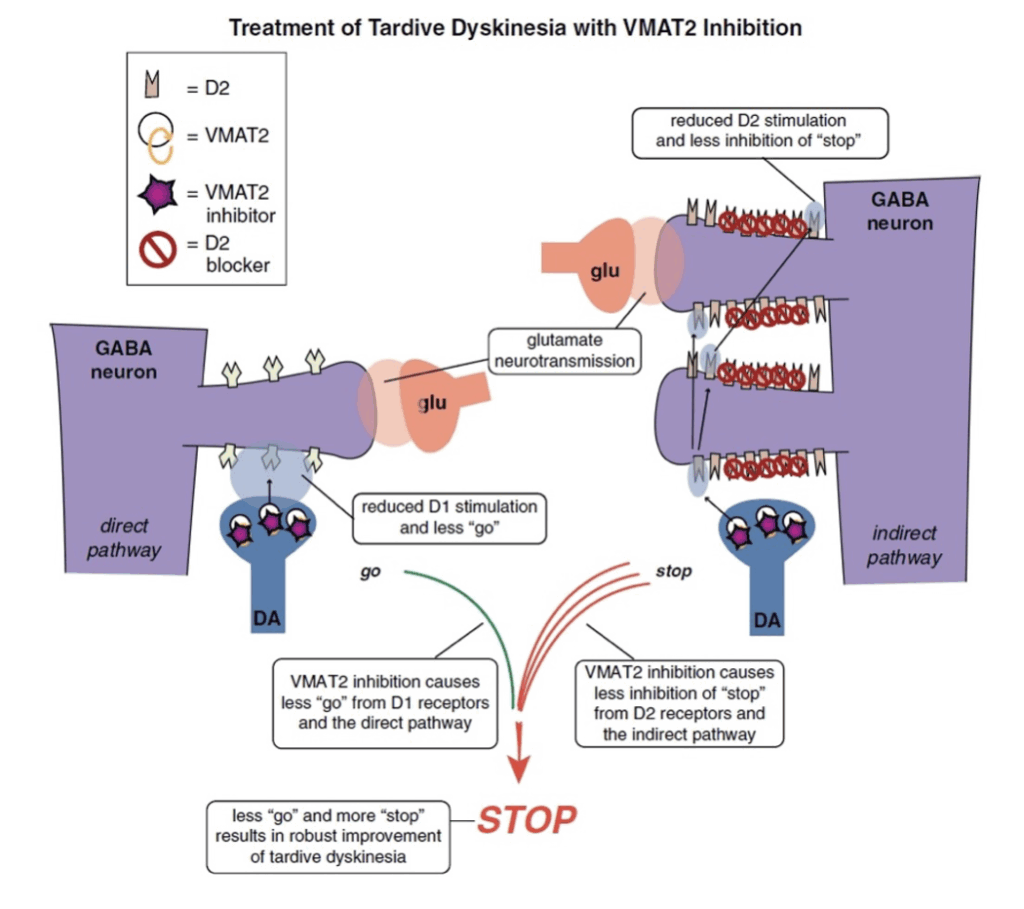
Image 1: Supersensitive D2 receptors
- “Direct central pathways” (seen on the left) – lead to hyper-kinesis.
- “Indirect central pathways” (seen on the right) – lead to hypo-kinesis.
- Tardive dyskinesia may be seen as an imbalance between “stop” and “go” signals from the motor striatum.
- D2 receptors in the indirect pathway of the motor striatum hypothetically react to chronic blockade by D2 antagonists by “learning” to have tardive dyskinesia with aberrant neuronal plasticity, resulting in super-sensitivity to dopamine. This leads to too much inhibition of “stop” signals coming from too much dopamine acting at upregulated D2 receptors in the indirect pathway (on the right), and unopposed
- D1 “go” signals coming from the direct pathway (on the left), thus resulting in involuntary hyperkinetic movements.
Image 2: VMAT2 inhibition
- This mechanism reduces dopamine stimulation without blocking D2 receptors.
- It will reduce overstimulation of D2 receptors in the indirect pathway (on the right), resulting in less inhibition of the stop signal there.
- It will also inhibit VMAT2 and reduce dopamine release in the direct pathway where “go” signals are amplified by dopamine at D1 receptors (on the left).
- Even though these D1 receptors and this direct extrapyramidal pathway may not be the site of pathology in TD, this pathway does drive “go” signals for movement, so lowering dopamine there by VMAT2 inhibition lowers “go” signals arising from the direct pathway (on the left). Combined with more “stop” signals from the indirect pathway (on the right), motor output to drive abnormal involuntary hyperkinetic movements can be robustly reduced by this combination of effects of dopamine depletion in both pathways.
References
Publications
- Schonecker M. Paroxysmale Dyskinesen als Megaphenwirung [Paroxysmal dyskinesia as the effect of megaphen]. Nervenarzt. 1957 Dec 20;28(12):550-3
- Faurbye A, Rasch PJ, Petersen PB, Brandborg G, Pakkenberg H. Neurological symptoms in pharmacotherapy of psychosis. Acta Psychiatr Scand. 1964;40(1):10-27
- Vijayakumar D, Jankovic J. Drug-Induced Dyskinesia, Part 2: Treatment of Tardive Dyskinesia. Drugs. 2016 May;76(7):779-87.
- Hauser RA, Factor SA, Marder SR, Knesevich MA, Ramirez PM, Jimenez R, Burke J, Liang GS, O’Brien CF. KINECT 3: A Phase 3 Randomized, Double-Blind, Placebo-Controlled Trial of Valbenazine for Tardive Dyskinesia. Am J Psychiatry. 2017 May 1;174(5):476-484.
- Stahl SM. Mechanism of action of vesicular monoamine transporter 2 (VMAT2) inhibitors in tardive dyskinesia: reducing dopamine leads to less “go” and more “stop” from the motor striatum for robust therapeutic effects. CNS Spectr. 2018 Feb;23(1):1-6
- Caroff SN. A new era in the diagnosis and treatment of tardive dyskinesia. CNS Spectr. 2023 Aug;28(4):401-415
FOAMed
- Nickson C. The man who blinked too much. LITFL
Fellowship Notes
MBBS DDU (Emergency) CCPU. Adult/Paediatric Emergency Medicine Advanced Trainee in Melbourne, Australia. Special interests in diagnostic and procedural ultrasound, medical education, and ECG interpretation. Co-creator of the LITFL ECG Library. Twitter: @rob_buttner
Educator, magister, munus exemplar, dicata in agro subitis medicina et discrimine cura | FFS |


