Ventricular Assist Device (VAD)
Reviewed and revised 20 July 2015
OVERVIEW
A Ventricular Assist Device (VAD) is a mechanical pump used to provide adequate cardiac output when heart failure is resistant to medical therapy
USES/INDICATIONS
Severe heart failure and cardiogenic shock (patients selected typically are NYHA Class IV, with EF <25% and VO2max <15) in the setting of:
- bridge to recovery — temporizing measure to optimize ventricular function while awaiting native ventricular function to return
(e.g. unable to wean off bypass, transient cardiomyopathy) - bridge to heart transplantation
- bridge to decision — temporizing measure until a decision can be made on one of the above
- destination therapy if not eligible for cardiac transplantation (not currently funded in Australia)
Contraindications
- Irreversible hepatic or renal failure not due to poor cardiac output
- Metastatic cancer
- Cerebral accident with neurological deficits
Relative contraindications
- Refractory tachyarrhythmias
- Active coagulopathy
- Active infection
- Mechanical cardiac valves
- Severe valvular regurgitation (especially aortic)
DESCRIPTION
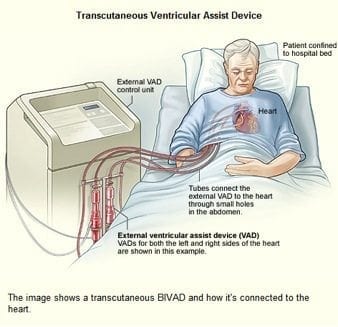
- a small tube that carries blood out of the heart into a pump then blood pumped back into the aorta (LVAD) or pulmonary artery (RVAD)
- can be continuous flow (cf-VAD) rather than pulsatile (generally older models); cf-VADs are smaller, quieter, and durable
- 2 main types of pumps currently used:
1. centrifugal (~2,500 rpm)
2. axial flow (~10,000 rpm) - may be right, left or biventricular
- either transcutaneous or implantable
- good control of cardiac output
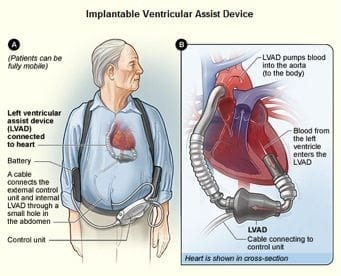
Components that may be present:
- inflow cannula — stationed inside the left ventricle to direct blood into the pump
- pump — located at the apex of the left ventricle and houses the impeller (magnetically levitated frictionless rotor)
- outflow graft — flexible, gel-impregnated conduit that directs blood from the pump to the ascending aorta
- controller — monitors pump function (flow, speed, power), controls pump speed and manages power supply, records pump data and alarms; displays battery life and function; data can be downloaded for analysis
- driveline — subcutaneously tunneled from the epigastrium or RUQ to the pump; contains wires that provide power and operating details to the pump from the controller
- stabilisation belt — supports and anchors the driveline at the exit site, reduces risk of infection, minimises tissue injury and promotes tissue growth
- batteries — patients carry at least 2 lithium batteries at all times; VAD can be recharged using standard power supply
METHOD OF INSERTION AND/OR USE
Site
- surgically placed
- varies with device e.g. HEARTMATE II has a subdiaphragmatic pocket
Flow
- LVAD — LV to aorta
- RVAD — RA or RV to pulmonary artery
- BiVAD — both of the above
OTHER INFORMATION
Key issues
- VADs are preload dependent and afterload sensitive
- requires anticoagulation to prevent pump or intracardiac thrombosis
- cardioversion is safe
- CPR can be performed (controversial)
- perioperative antibiotic prophylaxis is use following insertion (e.g. vancomycin and ceftazidime)
Assessment of VAD patients
- Most patients have tag located on their controller around their waist indicating what type of device it is, what institution put it in, and a number to call.
- pulse may be impalpable with cf-VAD (DBP increases)
- always auscultate (e.g. epigastrium/ precordium) to hear if the VAD is working
- assess blood pressure (typically aim for MAP 60-90 mmHg)
- may be difficult or impossible with standard non-invasive blood pressure measurement
- intra-arterial line is ideal
- can use brachial artery doppler to to detect loud contiuous noise during non-invasive blood pressure measure, this corresponds to the MAP
- check battery life and performance parameters on controller
- perform bedside echocardiography (see below)
- perform ECG — VAD pateints may tolerate otherwise lethal dysrhythmias (unless RV dysfunction occurs)
- assess for sepsis (including exit site swabs)
- also discuss patients with a VAD center
Key parameters (HEARTMATE II)
- rpm (pump speed — this is set according to ECHO results)
- flow (this is calculated from power, rpm and hematocrit/ blood viscosity) — paradoxically increased flow reading can be caused by obstruction as also leads to increased power requirement
- PI (pulsatility index — how much cardiac output is provided by the patient vs pump)
- power (required by the VAD — averaged over cardiac cycle
General VAD parameter ranges
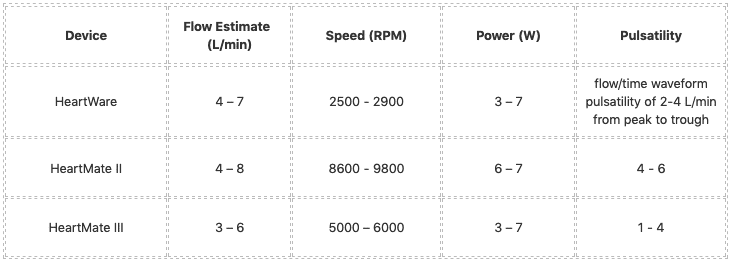
Lowest safe VAD operating speeds (e.g. for use during cardiac arrest)
- Heartware — Manually decrease speed to 1,800 rpm; No flow estimation given
- Heartmate II — Manually decrease speed to 8,000 rpm; No flow estimation if < 3L/min
- Heartmate III — Manually decrease speed to 4,000 rpm
Trouble-shooting power abnormalities (e.g. for HEARTMATE II) — note that pump speed (rpm) will remain constant unless there is a suction event:
- low power with low pulsatility index and steady pump speed
- causes: inflow/ outflow obstruction, LV failure, dysrhythmia, hypertension
- low power with normal or high pulsatility index
- suckdown
- high power with low pulsatility index fluctuating pump speed
- pump thrombosis, vasodilation, hypotension, initial response to exercise
- high power with high pulsatility index
- normal physiological response to increased demand; myocardial recovery; fluid retention
Echocardiography findings
- small RV suggests inadequate preload
- small LV suggests suckdown
- large RV and small LV suggests RV failure or pulmonary hypertension
- large RV and large LV suggests pump thrombosis/ obstruction
Causes of hypotension
- VAD-related:
- suckdown
- malposition
- pump failure/ obstruction (thrombosis, kinking, etc)
- excessive pump speed
- non-VAD-related:
- hypovolemia (dehydration or haemorrhage)
- cardiac tamponade
- aortic regurgitation
- dysrhythmias
- vasodilation
Causes of dysrhythmia
- local physical effects (e.g. malposition, suck down), electrolyte disturbance, ischemia, underlying cardiomyopathy
COMPLICATIONS
Main issues with LVAD post-insertion
- right heart failure (due to mechanical interdependence and haemodynamic reasons)
- bleeding/ clotting
- suckdown
Cardiovascular
- right heart dysfunction
- up to 20% develop acute right heart failure post-VAD
- aortic regurgitation
- often induced by LVAD
- dysrhythmia
- typically due to a “suction event” (where the inflow cannula contacts the ventricular septum), most common in the first month
- Suction events can be caused by hypovolemia, small ventricular size, or RV failure and are treated with fluid resuscitation and decreasing the LVAD speed
- CVA
- prevent with aggressive management of hypertension with ACEI/ B-blockers
Device-related
- suckdown
- due to right ventricular failure, hypovolaemia, tamponade, dysrhythmias, inflow cannula malposition
- device failure
- malposition/ migration
- mobility restrictions
Hematological
- bleeding (e.g. epistaxis, intracranial, intrathoracic)
- anticoagulation
- post-surgical bleeding
- intracranial haemorrhage (higher with axial flow pumps)
- acquired von Willebrand factor deficiency
- gastrointestinal angiodysplasia and AV malformations
- thromboembolism (higher with centrifugal pumps, see above)
- haemolysis
- check haptoglobin, free Hb, LDH, Hb in urine; consider inflow/ outflow obstructions and pump dysfunction
Infection
- VAD-specific infections
- pump and/or cannula infections, pocket infections, and driveline infections (usually Staphylococcus or Enterococcus; for pump pocket and deeper wound infections need to cover pseudomonas)
- VAD-related infections
- infective endocarditis, bloodstream infections, and mediastinitis
- non-VAD-related infections
- e.g. LRTI, UTI
VAD thrombosis
- dramatic increase in the rate of thrombosis since 2011 in the HeartMate II device (Starling et al, 2014)
- increase in pump thrombosis at 3 months after implantation from 2.2% to 8.4%
- median time from implantation to thrombosis was 18.6 months prior to March 2011, to 2.7 months after
- up to 50% mortality
- may present as
- power spikes or low pump flow alarms on the patient’s control box Pump (VAD) failure, recurrent/new heart failure, ltered mental status, hypotension (MAP < 65), or signs of peripheral emboli (including acute CVA), evidence of hemolysis (LDH > 1,500 mg/dL or 2.5-3 times the upper limit of normal, haemoglobinuria and elevated plasma free hemoglobin)
- management
- anticoagulation (e.g. heparin), may require thrombolysis/ thrombectomy/ surgical intervention
DEVICE TYPES
HeartMate I or XVE
- Use: Destination Therapy
- Flow Type: Pulsatile
- Pulse: Has pulse but may not match ECG rhythm
- Backup Method: Hand Pump
- Battery: 12volt MiMH – 10hrs
- Defib/Cardioversion: Use hand pump during defib/cardioversion
- Anticoagulation: patient on aspirin
HeartMate II
- Use: Bridge to transplant or destination therapy
- Flow type: axial flow
- Backup Method: No external method
- Pulse: No palpable pulse or BP. Doppler Only
- Battery: 14V Li-Ion – 10 hrs
- Defib/Cardioversion: No precautions necessary
- Anticoagulation: Warfarin
Thoratec VAD
- Use: Bridge to Transplant
- Flow Type: Patient will have pulse and BP but may not match ECG rhythm
- Backup Method: No external method
- Battery: 12V lead acid gel battery – 7.2 Ah – up to 3 hrs
- Defibrillation/Cardioversion: No precautions
- Anticoagulation: Warfarin
References and Links
Journal articles
- Felix SE et al. Continuous-flow left ventricular assist device support in patients with advanced heart failure: points of interest for the daily management. Eur J Heart Fail. 2012 Apr;14(4):351-6. PMID: 22308012.
- Partyka C, Taylor B. Review article: Ventricular assist devices in the emergency department. Emerg Med Australas. 2014 Apr;26(2):104-12. PMID: 24707998.
- Pratt AK, Shah NS, Boyce SW. Left ventricular assist device management in the ICU. Crit Care Med. 2014 Jan;42(1):158-68. PMID: 24240731.
- Rose EA, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001 Nov 15;345(20):1435-43. PMID: 11794191.
- Slaughter MS, et al; HeartMate II Clinical Investigators. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010 Apr;29(4 Suppl):S1-39. PMID: 20181499.
- Starling RC et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014 Jan 2;370(1):33-40. PMID: 24283197.
FOAM and web resources
- Blunt Dissection — Part man… Part machine (2014)
- ED ECMO — LVAD Problems and Troubleshooting (2014)
- EMCrit — Left Ventricular Assist Devices (LVADs) (2013)
- EM Docs — Ventricular Assist Device Management (2014)
- EM Nerd — The Adventure of the Resident Patient (2013)
- EP Monthly — The LVAD: Walking, Talking … and Pulseless by Stuart Swadron (2012)
- ICN — Podcast 105. SIN Talk: Nair on LVADs in ICU (2013)
- JEMS — Patients with a Ventricular Assist Device Need Special Considerations (2012)
- Maryland CC Project — Introduction to the Left Ventricular Assist Device (2013)
- Maryland CC Project — Ventricular Arrhythmias in the LVAD Patient (2014)
- Maryland CC Project — LVAD Troubleshooting – Low flow & power drops (2014)
- MyLVAD.com — EMS Field Guides
- UMEM Education Pearls — VAD thrombosis (2013)
- Video lectures by Arie Blitz — VAD lecture and Heartmate II LVAD insertion (2011)

Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC