Funtabulously Frivolous Friday Five 296
Just when you thought your brain could unwind on a Friday, you realise that it would rather be challenged with some good old fashioned medical trivia FFFF, introducing the Funtabulously Frivolous Friday Five 296
Question 1
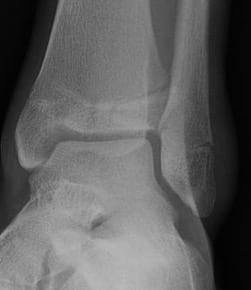
Where would you find a Tillaux fracture?
Reveal the funtabulous answer
In a teenager’s ankle
Tillaux fractures are uncommon fractures that occur most commonly near skeletal maturity, usually 12-14 in girls and 15-18 in boys. The anterolateral portion of the epiphysis is avulsed when external rotation places stress on the anterior-inferior talofibular ligament (ATFL). The mechanism is either through lateral rotation of the foot or by medial rotation of the leg on a fixed foot. The medial portion of the growth plate fuses earlier then the lateral portion.
Given the subtle nature of many of these fractures they are commonly missed. Plain radiography can distinguish a Tillaux from a true triplanar fracture, however may be insufficient to properly appreciate the level of distraction of the avulsed segment, thus a CT is usually required to aid management and prognostication.
Paul Jules Tillaux (1834 – 1904) first described this particular fracture in 1872. He performed experiments on cadavers and found that stress to the anterior inferior tibiofibular ligament could lead to this type of avulsion fracture, which today is termed the Tillaux fracture
References
- Cadogan M. Eponymous ankle and talus injuries. LITFL
- Howard J. Tillaux fracture. LITFL
- Tillaux PJ. Recherches cliniques et expérimentales sur les fractures malléolaires. [Reported by Leon Gosselin]. Bulletin de l’Academie de médecine. 1872; 21: 817-826
- Santos-Pereira R et al. Painful Nonunion after Missed Juvenile Tillaux Fracture in an Athlete – Case Report and Description of a New Fixation Technique. 2018; 8(5): 7-10
Question 2
In the year 2020, it is estimated that the doubling time of biomedical knowledge will be:
- 73 days
- 2.6 years
- 6-7 years
Reveal the funtabulous answer
73 days
The rate of health data generation is increasing almost exponentially. By some recent estimates, as of 2020 people and health systems in the U.S. and around the world will generate data 50 times more rapidly than they did at the start of the decade.
Biomedical knowledge generation worldwide has been accelerating at a correspondingly profound rate. One estimate suggests that the doubling time of biomedical knowledge–which stood at 50 years in 1950 and 7 years in 1980–will decrease to an estimated 73 days by 2020
Reference:
- Friedman CP, Rubin JC, Sullivan KJ. Toward an Information Infrastructure for Global Health Improvement. Yearb Med Inform. 2017 Aug;26(1):16-23
Question 3
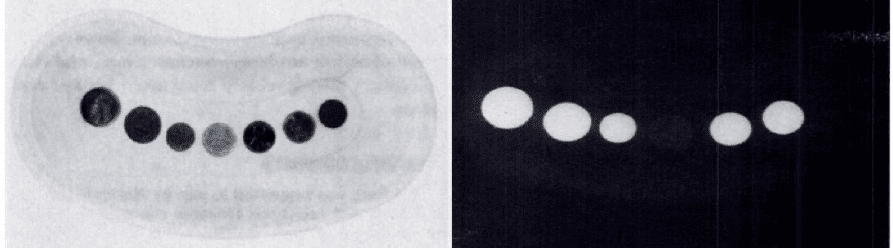
In 1973-4 the United States Mint proposed the release of an aluminium coin to replace the existing bronze (95% copper 5% zinc) pennies, due to the rising costs of copper at the time. The coin was opposed by US paediatricians at the time, why?
Reveal the funtabulous answer
Aluminium is very difficult to find on x-rays!
Children will put damn near anything in their mouths, and not infrequently either swallow them, or sometimes aspirate. The US paediatricians were worried that the radiodensity of aluminium (atomic no. 13) was too close to the density of soft tissue (average atomic weight 8-9) that reliably locating aluminium foreign bodies on x-ray would be near impossible.
The coins were not put into circulation due to these successful protests, which were joined by protest from the vending machine industry.
Some coins were produced for distribution amongst mint staff and senators whilst discussing the proposed change. Almost all of them were recovered by the mint but for a couple. The only coin (dubbed 1974-D) not recovered by the mint was offered for sale for up to US$2,000,000.00, however it was then surrendered back to the mint as part of the settlement terms of a lawsuit.
** Coins from left = aluminium bronze, zinc, zinc, aluminium, bronze, copper plated, fibre.
To answer the question of whether aluminium is truly invisible on x-ray, a 2005 study on cadavers demonstrated that aluminium is indeed visible, although with difficulty. Aluminium can tabs were placed at the upper oesophagus/posterior pharyngeal area in ten randomly selected cadavers. Anterior-posterior and lateral radiographs were performed before and after placement.
The can tabs were visible on lateral x-rays, and not visible on AP films for the superimposition of the vertebral bodies.
Reference:
- Dorst JP, Reichelderfer TE, Sanders RC. Radiodensity of the Proposed New Penny. Pediatrics. 1982; 69(2)
- Valente JH et al. Aluminum foreign bodies: do they show up on x-ray? Emerg Radiol. 2005 Dec;12(1-2):30-3
- 1974 aluminum cent. Wikipedia
Question 4
Which medication contains a ketone combined with an amine?
Reveal the funtabulous answer
Ketamine
In the post World War 2 strive for better pharmacological agents, in particular the search for the ‘ideal’ anaesthetic agent, several useful agents were discovered. Phencyclidine (PCP) was the first drug derived in Detroit in the mid-1950s. It was quickly appreciated to be a potent analgesic but ultimately unsuitable for human use after trials in people showed ~15% of people had persistent (up to 12 hours) severe agitation / psychosis.
In 1962, Calvin Stevens produced ‘2-(O-chlorophenyl)-2-methyl-amino cyclohexanone’ which he found to produce an excellent, short acting, anaesthesia. He named it “ketamine” because it comprised a ketone bound with an amine. The first human trial was in 1964, on volunteer prisoners of the Jackson Prison in Michigan. Participants described their experiences as floating in space and having no feelings in the limbs at all.
Whilst debating ways to describe their data, the authors considered, then avoided, the term ‘schizophrenomimetic’, as they felt it would likely be a commercial disaster. The three researchers were about to coin the term ‘dreaming’ to describe the peculiar anaesthetic state, when fortunately a wife of one researcher suggested the term ‘dissociative anaesthetic’ to encapsulate that many patients appears disconnected.
Reference:
- Mion G. History of anaesthesia: The ketamine story – past, present and future. Eur J Anaesthesiol. 2017 Sep;34(9):571-575
Question 5
What is the Fink effect?
Reveal the funtabulous answer
Diffusion hypoxia at the end of nitrous sedation
In 1955, Bernard Raymond Fink (1914 – 2000) published “Diffusion anoxia” – a study of eight patients undergoing gynaecologic surgery with 75% nitrous oxide – 25% oxygen mix. Fink noted that the patients’ oxygen saturations dropped 5-10% upon cessation of nitrous oxide and ventilation of room air. This lead to the conclusion that oxygen should routinely be given at the end of sedations.
The underlying mechanism of diffusion hypoxia is that given nitrous oxide is poorly soluble in blood, once the driving force of high alveolar nitrous oxide concentration is removed, its will diffuse rapidly back from the blood into the alveolar space, diffuse and possibly displace end alveolar O2.
The significance of the Fink effect has since been downplayed through other work that suggests airway obstruction, alveolar atelectasis and other respiratory irregularities play a larger role than diffuse hypoxia. However, given its sound physiologic underpinning and absence of evidence that rule out the phenomenon. It’s still prudent to place some O2 on anyone you’ve just given a nitrous sedation to.
References:
- Fink BR. Diffusion anoxia. Anesthesiology. 1955; 16(4): 511-9.
- Cheney FW. An Early Example of Evidence-based Medicine: Hypoxemia due to Nitrous Oxide. Anesthesiology 2007; 106: 186-188

FFFF
Funtabulously Frivolous Friday Five
Dr Mark Corden BSc, MBBS, FRACP. Paediatric Emergency Physician working in Northern Hospital, Melbourne. Loves medical history and trivia...and assumes everyone around him feels the same...| LinkedIn |



Wonderful Questions!
I have a comment on the nomenclature of “ketamine”. It was named as such because it contains a “ketone group: RC(=O)R’ ” and an amine group.
It does NOT contain a “ketone body” as mentioned in the answers. There are 3 ketone bodies (acetone, acetoacetate, and 3-beta-hydroxybutyrate), none if which are part of “ketamine”.
Thanks
Hi Ali,
Thanks for the feedback! Will rectify ASAP.
Cheers,
Mark