Hyponatraemic Seizures
aka Metabolic Muddle 012
A 10 year-old boy with a history of enuresis was BIBA to the ED after a first episode generalized tonic-clonic convulsion. He seemed tired in the morning but still attended his inter-school sports competition. While getting ready to compete he collapsed and had a self-limiting seizure (5 minutes duration).
He was afebrile and had GCS 13 (E3 V4 M6) (fluctuating) for about 4 hours without improvement. Pupils were equal and reactive and he had no focal neurological deficits. He vomited 5 times during this time but was clinically euvolemic. His CT head was normal. After returning from the scan he had another self-limiting seizure that lasted 2 minutes.
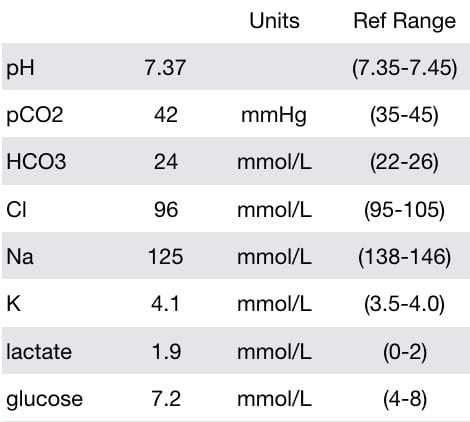
He had the following laboratory test results (between seizures):
Questions
Q1. What are the laboratory abnormalities and how do they relate to his presentation?
Answer and interpretation
The laboratory findings are:
- Hyponatremia (125 mmol/L)
- Borderline low chloride — chloride may be lost due to vomiting.
- Mildly increased lactate — hyperlactemia is typically found following a seizure, in this case it may actually be normalising after the first seizure
- Mildly increased glucose — glucose is commonly elevated after a brief seizure, or in the early stages of status, due to a catecholamine-mediated ‘stress response’.
Symptoms of hyponatremia do not necessarily correlate well with the degree of hyponatremia. Expected symptoms are typically:
- <125-130 mmol/L – nausea and malaise
- <115-120 mmol/L – headache, lethargy, obtundation, seizures, coma, respiratory arrest, noncardiogenic pulmonary edema.
However, significant symptoms may be found at higher levels depending on the ‘starting concentration’ and the rate of decrease.
For instance, worse symptoms are more likely if sodium rapidly drops from 140 mmol/L to 125 mmol/L than if there is a slow decrease from 130 mmol/L to 115 mmol/L.
Q2. How can you determine if the laboratory abnormality identified in Q1 is contributing to the patient’s symptoms?
Answer and interpretation
Determine if the patient’s neurological status improves by increasing his plasma sodium (and osmolality).
The child was administered 3mL/kg of 3% saline over 30 minutes. Immediately following this infusion he was alert with a GCS 15 and had no further vomiting. His only complaint was a mild headache that improved with paracetamol.
This response to treatment suggests (but does not prove) that he was significantly symptomatic with a plasma sodium of 125 mmol/L.
Finally, it is important to remember is that hyponatremia is not a diagnosis – we still don’t know whys he was hyponatremic.
Q3. Is it safe to perform the measure taken in Q2?
Answer and interpretation
There may be a reluctance to administer hypertonic saline due to the fear of the dreaded complication of cerebral pontine myelinolysis (perhaps better called osmotic demyelination syndrome – more than the pons may be involved). This complication may occur with the excessive correction of hyponatremia in patients that have chronic severe hyponatremia (e.g. Na 110-115 for at least 2 days).
Chronicity is important because the brain adapts to hyponatremia by extruding intracellular osmolytes to guard against cerebral edema. The adaptation process occurs over about 2 days, and until it occurs correcting hyponatremia is safe.
In acute symptomatic hyponatremia the risk of osmotic demyelination syndrome from rapid correction of hyponatremia is minimal.
Hypertonic saline should be administered to patients with significant symptoms (e.g. altered mental state, seizures, coma, noncardiogenic pulmonary edema) of hyponatremia, regardless of the sodium level. Usually the aim is to increase the sodium by 1-1.5 mmol/h for 2 or 3 hours, and a small rise can markedly improve symptoms.
In general, sodium should not be increased by more than 10-12mmol over 24h, and 18 mmol/L over 48h. Lower rates are advised for high risk patients (e.g. chronic hyponatremia in the context of malnutrition, alcoholism or advanced liver disease)
The daily rate of increase is more important than the hourly rate, in terms of risk of osmotic demyelination syndrome. So, once the symptoms have improved the rate of correction should be slowed.
Further laboratory test results were obtained:
Serum cortisol 1100 nM (60-420)
TFTs were normal
Osmolality plasma 265 mmol/kg L (275-295)
Spot urine sodium 209 mM
Spot urine osmolality 681 mmol/kg (50-1200)
Q4. What do these test results suggest?
Answer and interpretation
These results are consistent with the syndrome of inappropriate anti-diuretic hormone secretion (SIADH).
ADH (aka vasopressin) promotes water reabsorption from the collecting ducts of the kidney by activating the vasopressin V2 receptor. This stimulates the translocation of aquaporin-2 water channels from intracellular sites to the luminal membranes of the principal cells of the collecting duct. The end result is concentrated ‘water-poor’ urine and dilute ‘water-rich’ blood.
Features of SIADH include:
- Low plasma osmolality
- urine osmolality > plasma osmolality (usually >300-400 mosmol/kg)
- Urine sodium concentration usually >40 meq/L
- Normal acid-base and potassium balance
- Normal renal, liver, adrenal and thyroid function
- Diuretics are not in use
- improves with water restriction
Although these results are suggestive of SIADH, we still do not have the underlying diagnosis… what is the cause of this ‘SIADH’?
Q5. What are the possible causes in this case?
Answer and interpretation
The possible causes of SIADH include:
- Any CNS disorder –
stroke, hemorrhage, infection, trauma, and psychosis - Pulmonary disorders –
lung cancer, pneumonia, bronchiolitis, pneumothorax, asthma, etc. - Ectopic ADH secretion by a tumour –
lung cancers (especially small cell lung cancers), and less commonly:
cancer of the duodenum or pancreas, head and neck cancer, and olfactory neuroblastomas - Major surgery –
especially thoracic or abdominal. - Drugs –
many drugs, including:
SSRIs, ecstasy, antipsychotics like haloperidol, antiepileptics (e.g. valproate and carbamazepine), MAOIs, NSAIDs, opiates, chemotherapy (e.g. cyclophosphamide, vincristine), amiodarone, bromcriptine, ciprofloxacin…
However, the child in this case did not have SIADH…
Some conditions may mimic SIADH. These include:
- Hereditary vasopressin receptor abnormalities (‘nephrogenic SIADH’)
- Cerebral salt wasting (classically in neurological disorders such as subarachnoid haemorhage — it resembles SIADH but the patient is hypovolemic and is responsive to normal saline rather than water restriction)
- Exogenously administered vasopressin agonists such as vasopressin, desmopressin and oxytocin.
Do you remember from the history that this child had problems with enuresis?
The child had started using a nightly nasal spray of desmopressin to treat his enuresis about 4 days prior to his ED presentation. This exogenously administered analogue of ADH resulted in hyponatremia mimicking SIADH, probably exacerbated by increased water intake prior to his sports competition.
Over the next 12-24 hours he had a large diuresis and his laboratory values all normalized. He remained well.
Further Reading
References
- Boetzkes S, Van Hoeck K, Verbrugghe W, Ramet J, Wojciechowski M, Jorens PG. Two unusual pediatric cases of dilutional hyponatremia. Pediatr Emerg Care. 2010 Jul;26(7):503-5. PMID: 20622630.
- Douglas I. Hyponatremia: why it matters, how it presents, how we can manage it. Cleve Clin J Med. 2006 Sep;73 Suppl 3:S4-12. PMID: 16970147
- Fleming JD, Babu S. Images in Clinical Medicine — Central Pontine Myelinolysis. N Engl J Med 2008; 359:e29 [Free fulltext online]
- Nickson C. Environmental Enigma 001: Exercise-associated hyponatremia. LITFL.
- Robson WL, Leung AK, Norgaard JP. The comparative safety of oral versus intranasal desmopressin for the treatment of children with nocturnal enuresis. J Urol. 2007 Jul;178(1):24-30. Epub 2007 May 11. Review. PMID: 17574054
- Schrier RW, Bansal S. Diagnosis and management of hyponatremia in acute illness. Curr Opin Crit Care. 2008 Dec;14(6):627-34. PMID: 19005303
- Gussow L. Desmopressin and the “pis-quiz”: unexpected causes of low sodium. The Poison Review.
- Van de Walle J, Van Herzeele C, Raes A. Is there still a role for desmopressin in children with primary monosymptomatic nocturnal enuresis?: a focus on safety issues. Drug Saf. 2010 Apr 1;33(4):261-71. PMID: 20297859.
- Weingart S. EMCrit Podcast 39 — Hyponatremia.
LITFL
- CCC – Hypernatraemia
- CCC – Hypernatraemia Mind map (PDF)
- CCC – Hyponatraemia
- CCC – Hyponatraemia Mind map (PDF)
- CCC – Hyponatraemia Interpretation (PNG)
- CCC – SIADH – SIADH DDx
- CCC – Diabetes Insipidus Central – Diabetes Insipidus DDx
- Case – Exercise-associated Hyponatremia
- Case – Seizures, hyponatremia and ADH
CLINICAL CASES
Metabolic Muddle
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC