Irukandji Syndrome Territory-style
aka Toxicology Conundrum 009
You are working as a locum doctor in the Northern Territory.
Your patient is a 32 year-old Indonesian man who says he was stung while hauling in a net on an offshore fishing vessel. Initially he had stinging sensations in his legs and back with the appearance of visible welts. His symptoms progressed to excruciating abdominal, chest, and limb pains, followed by vomiting. He arrived at your hospital 16 hours after the onset of symptoms and complained of ongoing pain and dyspnoea. He had tachycardia (100/min), hypertension (170/80 mmHg), tachypnea (28/min), pulse oximetry of SO2 95% on air, and bibasal lung crepitations. His electrocardiograph showed widespread non-specific ST-T abnormalities.
You initiated treatment with morphine IV, chlorpromazine IV, and metoclopramide IV. The patient then had a two minute episode of non-sustained ventricular tachycardia. As he remains distressed, you treat him with magnesium sulfate (10 mmol IV over 15 minutes, followed by a further 10 mmol IV over 2 hours).
Questions
Q1. What is the diagnosis? How did you make this diagnosis?
Answer and interpretation
This is a clinical diagnosis, there are no confirmatory laboratory investigations available.
Key features include:
- Recent contact with seawater
- A relatively innocuous initial sting
- A delayed systemic syndrome that progresses over minutes-to-hours, characterized by pain in multiple regions distant to the sting site and other symptoms such as nausea and vomiting, and restlessness. Features of a “catecholamine excess” state, such as tachycardia and hypertension, are common.
(see also Toxicology Conundrum 008)
Q2. What is the cause of this condition?
Answer and interpretation
Irukandji syndrome is a poorly understood condition characterized by severe pain, a hypercatecholaminergic state, and the potential for life-threatening cardiovascular complications. It is caused by jellyfish envenoming.
Carukia barnesi, a thumbnail-sized carybdeid (four-tentacled box jellyfish), is the only jellyfish conclusively proven to cause Irukandji syndrome (thanks to a certain Dr. Jack Barnes…). However, it is almost certain that other jellyfish also cause Irukandji syndrome, perhaps even “non-carybdeids”.
Carukia barnesi is rare or non-existent in Western Australian and Northern Territory waters where the syndrome is well described. In the Northern Territory most stings occur in deep water and are associated with different seasonal and environmental features to Irukandji syndrome in North Queensland. Also, the sting lesions are not always consistent with classic descriptions.
Some of the other suspects are detailed in this table.
Q3. Where does this condition occur?
Answer and interpretation
Irukandji syndrome is well described throughout the northern coastal regions of Australia.
Its recognized distribution extends from near Fraser Island in Queensland across the Northern Territory to Exmouth in Western Australia. However, cases probably occur further south in Queensland, and as far south as Coral Bay in Western Australia.
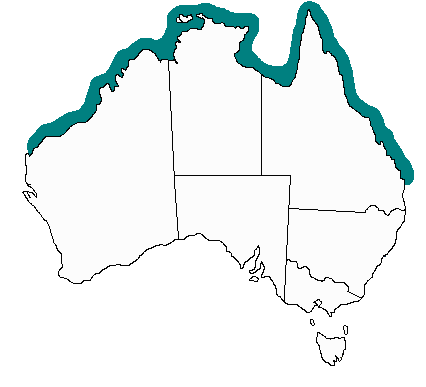
There have also been sporadic reports of Irukandji syndrome, and Irukandji-like syndromes, from around the world; including places as far flung as Victoria in Australia, Hawaii, the Caribbean (e.g. Florida), Thailand, and Papua New Guinea. The causative jellyfish species in these cases is unknown.
Q4. What is the rationale for treatment with magnesium?
Answer and interpretation
The use of magnesium in Irukandji syndrome remains an unproven and experimental therapy. It is often used in patients with symptoms refractory to conventional measures.
- In a small case series of 10 patients the use of magnesium sulfate was associated with a mean reduction in pain scores (out of 10) from 8.7 to 2.8. However, there are also reports of failure of magnesium therapy, and the pain can be cyclical making cause-and-effect difficult to assess. Definitive investigation of the effectiveness of magnesium therapy in treating Irukandji syndrome requires a well designed randomized controlled trial. However the widespread acceptance of magnesium in North Queensland may mean that such a study will never be performed.
Why might we expect magnesium therapy to be useful as a treatment for Irukandji syndrome?
- The Irukandji toxin is hypothesized to stimulate catecholamine release and may have other direct effects, such as cardiotoxicity, resulting from sodium channel activation and possibly other mechanisms. Magnesium is known to have a depressant effect on the release of catecholamines, has anti-arrhythmic properties, and has a vasodilatory effect.
- Many of the manifestations of Irukandji syndrome are shared by other conditions for which magnesium is used with apparently good effect. It has been used safely in the treatment of eclampsia, acute asthma, and the “catecholamine excess” condition of phaeochromocytoma. It has also been used to treat muscle spasms in tetanus and autonomic instability in autonomic dysreflexia. Studies have also shown that magnesium has opioid-sparing effects in pain control in peri-operative settings.
Q5. What are the possible reasons for this man’s dyspnoea?
Answer and interpretation
The possibilities include:
- Acute pulmonary edema – this is highly likely given the patient’s prolonged symptoms, the presence of bilateral crepitations, and the evidence of cardiotoxicity (non-sustained ventricular tachycardia)
- Pain – patients with Irukandji syndrome commonly report difficulty breathing due to chest and abdominal pain
- Aspiration pneumonitis – this may result from repeated vomiting caused by Irukandji syndrome
- Bronchospasm – occasionally Irukandji syndrome is mistaken for anaphylaxis
- Upper airway compromise from a sting on the face or neck
- Exacerbation of an underlying illness
Q5. Describe your management plan.
Answer and interpretation
- Resuscitation –
Pulmonary oedema is a potential early life‑threat that requires immediate intervention:
– high-flow oxygen (15 L/min via non-rebreather mask)
– consider non-invasive positive pressure ventilation, or intubation and ventilation if indicated.
– consider ipratropium nebulisers if bronchospasm is suspected. Beta2-agonists should be avoided due in the presence of endogenous catecholamine release. - Supportive care and monitoring –
– Instead of morphine, administer IV fentanyl (0.5-1.0 microgram/kg/dose) repeated every 10 minutes until appropriate analgesia is achieved. Large doses may be required (e.g. 200-300 microgram). Fentanyl has a better cardiac stability profile.
– Control hypertension refractory to opioid analgesia with an intravenous infusion of glyceryl trinitrate (50 mg in 100 mL starting at 6 mL/minute; 1-4 microgram/kg/minute in children) titrated to achieve a systolic blood pressure <160 mmHg. Treatment can be rapidly withdrawn if hypotension ensues.
– Magnesium sulfate (already administered).
– Consider invasive monitoring (central venous access and cardiac output monitoring such as PiCCO or a pulmonary artery catheter) - Investigations – required to rule out other diagnoses and to detect/ confirm cardiovascular complications:
ECG and continuous monitoring (arrhythmia, ischemic changes, conduction blocks)
Chest radiograph (acute pulmonary edema, aspiration, coexistent disease)
FBC, UEC, troponin, CK (cardiotoxicity, multi-organ dysfunction, coexistent disease)
Echocardiography (cardiomyopathy) - Decontamination, Enhance elimination, Antidotes – nil
- Disposition –
Admission to HDU/ ICU level care until resolution of the transient cardiomyopathy caused by Irukandji syndrome.
References
- Irukandji Syndrome LITFL
- Jack Barnes (1922-1985) and the Irukandji enigma
- Toxicology Conundrum 008
- Corkeron MA. Magnesium infusion to treat Irukandji syndrome. Med J Aust. Apr 21 2003;178(8):411. [fulltext]
- Corkeron M, Pereira P, Makrocanis C. Early experience with magnesium administration in Irukandji syndrome. Anaesth Intensive Care. Oct 2004;32(5):666-669. [abstract]
- Grady JD, Burnett JW. Irukandji-like syndrome in South Florida divers. Ann Em Med 2003;42:763-766.[abstract]
- Fawcett WJ, Haxby EJ, MAle DA. Magnesium: physiology and pharmacology. Brit J Anaes 1999; 83:302-320. [abstract]
- Huynh TT, Seymour J, Pereira P, Mulcahy R, Cullen P, Carrette T, Little M. Severity of Irukandji syndrome and nematocyst identifcation from skin scrapings. Med J Aust 2003;178:38-41. [fulltext]
- Little M, Mulcahy RF. A year’s experience of Irukandji envenomation in far north Queensland. Med J Aust 1998;169:638-41. [fulltext]
- Little M, Mulcahy RF, Wenck DJ. Life-threatening cardiac failure in a healthy young female with Irukandji syndrome. Anaesth Intensive Care. Apr 2001;29(2):178-180. [abstract]
- Little M. Failure of magnesium in treatment of Irukandji syndrome. Anaesth Intensive Care. Aug 2005;33(4):541-542.
- Little M, Pereira P, Carrette T, et al. Jellyfish responsible for Irukandji syndrome. QJM. Jun 2006;99(6):425-427. [fulltext]
- Little M, Pereira P, Mulcahy R, et al. Severe cardiac failure associated with presumed jellyfish sting. Irukandji syndrome? Anaesth Intensive Care 2003;31(6):642-647. [abstract]
- Macrokanis CJ, Hall NL, Mein JK. Irukandji syndrome in northern Western Australia: an emerging health problem. Med J Aust 2004;181:699-702. [fulltext]
- Nickson CP, Waugh EB, Jacups SP, Currie BJ. Irukandji syndrome case series from Australia’s tropical Northern Territory. Ann Emerg Med. 2009 Sep;54(3):395-403 [PMID 19409658]
- Tibballs J, Li R, Tibballs HA, Gershwin LA, Winkel KD. Australian carybdeid jellyfish causing “Irukandji syndrome”. Toxicon. 2012 May;59(6):617-25. Epub 2012 Feb 14. PubMed PMID: 22361384.

CLINICAL CASES
Toxicology Conundrum
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC
