Second disease
Scarlet fever
Scarlet fever is an acute, infectious illness caused by toxin-producing strains of Group A β-haemolytic Streptococcus (GABHS), primarily Streptococcus pyogenes. It predominantly affects children aged 5–15 years and manifests with a sore throat, fever, and a characteristic fine, red, sandpaper-like rash.
The disease results from a hypersensitivity reaction to streptococcal erythrogenic (pyrogenic) exotoxins, which act as superantigens. Scarlet fever can arise from pharyngitis or impetigo, but pharyngeal infection is far more common in temperate climates.
Transmission occurs via respiratory droplets, and the incubation period is typically 1–4 days. The illness was historically associated with high morbidity and mortality before the antibiotic era. While the widespread use of antibiotics has significantly reduced the burden in high-income countries, scarlet fever remains a public health concern in areas with limited healthcare access and continues to show periodic surges globally, including recent outbreaks in the UK and East Asia.
The development of the Dukes classification in 1900 designated scarlet fever as the second of the six classic childhood exanthems. It remains of clinical importance due to its overlap with other febrile rash illnesses and its potential for post-streptococcal complications, such as acute rheumatic fever and glomerulonephritis.
Prompt recognition and antibiotic treatment are essential for symptom resolution, reduction of transmission, and prevention of complications.
Synonyms of second disease: Scarlet fever, scarlatina, scarlatina anginosa, rossaniam, rossaliam, epidemic malignant purpura, rossalia of Ingrassias, male da scarlatina, die Deittelen, febris rubra, morbilli ignei, erysipelata, universal erysipelas
English physician Clement Dukes (1845–1925) introduced the numbering system for childhood exanthems in 1900. He categorised them by clinical presentation into: First: measles; Second: scarlet fever; Third: rubella; and Fourth: Filatov-Dukes disease. Later additions – Fifth: erythema infectiosum (1905, Cheinisse); and Sixth: roseola infantum (1910, Dreyfus)
Clinical manifestations
General features: sudden-onset fever, sore throat, tender cervical lymphadenopathy, headache, nausea, vomiting, anorexia, myalgia, and malaise. Coryza typically absent.
Pharyngitis: erythematous tonsillopharynx with exudates and petechiae on the palate (classic strep throat).
Rash: appears 24–48 hours after fever onset. Begins on neck, chest, axillae, groin → spreads body-wide over 24h.
- Fine, red, sandpaper-like, with confluent areas described as “sunburn with goose pimples”
- Blanches on pressure; lasts ~5–6 days; desquamation may follow, especially on hands/feet/toes/fingers
- lasting up to 6 weeks.
Key Signs
- Filatov mask: circumoral pallor with flushed cheeks; sparing of nasolabial folds (Filatov triangle).
- Pastia sign: linear petechiae in skin folds (antecubital fossa, axilla, groin); may precede rash and persist as pigmented lines post-desquamation. [Also: Pastia–Grozovici sign, Thomson sign]
- Strawberry tongue:
– White phase: white coating with protruding red papillae
– Red phase: glistening red tongue with prominent papillae after ~5 days
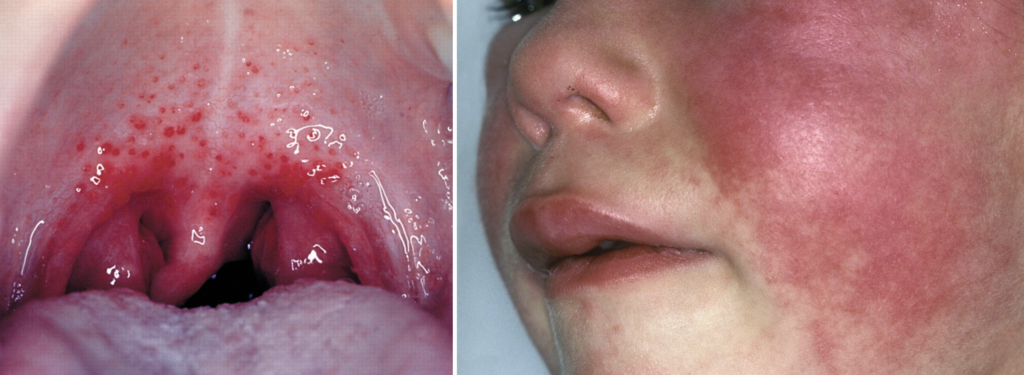
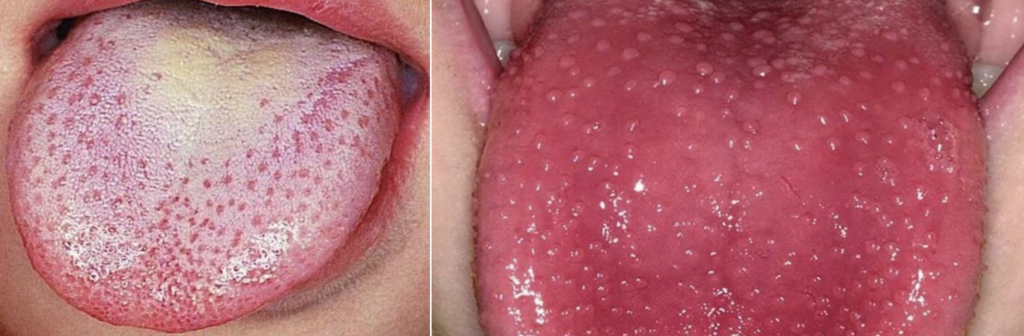
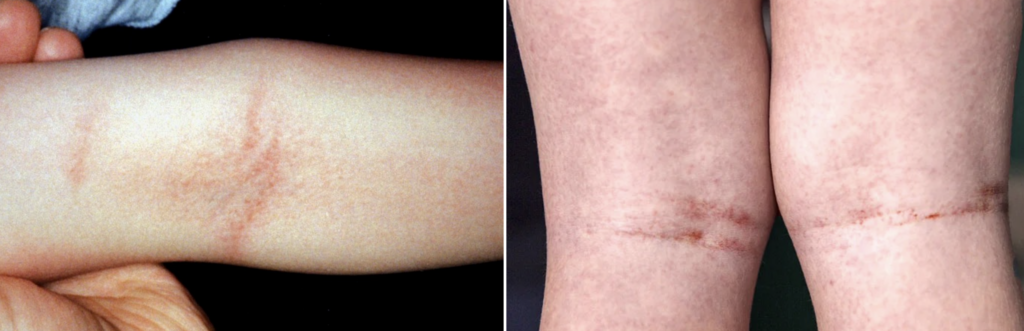
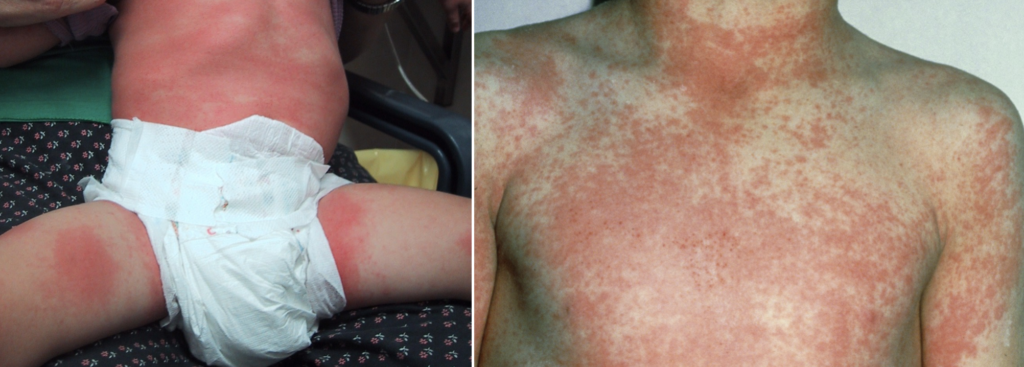
Diagnosis
Primarily clinical; supported by:
- Throat/tonsil swab for GABHS culture or rapid antigen test
- ASO or anti-DNase B titres (for post-infectious complications)
Treatment
Antibiotics rapidly relieve symptoms and prevent acute rheumatic fever when started within 9 days.
- First-line: Penicillin V or G
- Penicillin-allergic: Clarithromycin, Azithromycin, Clindamycin
- Non-type I allergy: Cephalosporins
Differential diagnosis
- Infectious mimics: viral hepatitis (early), mono, Kawasaki disease, TSS, measles, rubella
- Drug eruptions: sulfonamides, penicillin, streptomycin, quinine, atropine — often involve mucosal erosions
Complications
- Suppurative: otitis media, pneumonia, pericarditis, meningitis, hepatitis
- Non-suppurative:
- Post-streptococcal glomerulonephritis (PSGN)
- Acute rheumatic fever (ARF) → rheumatic heart disease
- PANDAS: controversial entity linking GABHS with autoimmune neuropsychiatric flares (e.g., OCD, tics)
History of second disease – scarlet fever
1553 – Giovanni Filippo Ingrassias (1510-1580), first professor at Naples and celebrated anatomist provided first major medical description of scarlet fever. He differentieated it from measles and chickenpox in De tumoribus praeter naturam.
…spots very large and small, fiery and red, scarcely raised…distinct from erysipelas, so that the whole body appears as if on fire [red hot].
Some there are, who think that measles is the same as rossalia, but we have often seen that the two affections are distinct, trusting in our own eyes and not merely in the description of others
1565 – Johann Weyer recognised scarlatina anginosa as a distinct febrile illness with sore throat.
1635 – Daniel Sennert (1572-1637) described an epidemic of “rossalia” at Wittenberg, echoing Ingrassias. First to document:
- Desquamation: epidermide squamarum instar decidente
- Arthritis: dolorem et ruborem ut in arthriticis
- Post-scarlatinal oedema and ascites.
Sennert’s insights were based partly on correspondence with his son-in-law Michael Döring (1582-1644), who described a Polish epidemic (1625)
1675 – Thomas Sydenham (1624-1689) opularised the term scarlatina (Febre Scarlatina), distinguishing it from measles:
Scarlet fever may appear at any season. Nevertheless it oftenest breaks out towards the end of the summer, when it attacks whole families at once, and more especially the infant part of them. The patients feel rigors and shivering just as they do in other fevers. The symptoms, however, are moderate. Afterwards, however, the whole skin becomes covered with small red maculae thicker than those of measles, as well as broader, and redder and less uniform. These last for two or three days and then disappear. The cuticle peels off and branny scales remain lying on the surface like meal. They appear and disappear two or three times
Though credited, the term was in vernacular use earlier; Samuel Pepys mentions “a Scarlett fevour” in his diary (1664)
My little girle Susan is fallen sicke of the meazles, we fear, or, at least, of a Scarlett fevour.
Pepys November 10th, 1664
1826 – Pierre-Fidèle Bretonneau (1778–1862) separated scarlet fever from diphtheria by introducing the term diphthérite. Armand Trousseau (1801-1867) later clarified with the the aphorism “La scarlatine n’aime pas le larynx”
1836 – Richard Bright (1759-1858) linked scarlatina to glomerulonephritis — a foundational observation in the history of Bright’s disease. He stated that “scarlatina has apparently laid the foundation for the future mischief.”
1874 – Theodor Billroth (1829-1894) provided the first description of streptococcal infection when he described the organism in cases of erysipelas and wound infections. He described “small organisms (Kettenkokken) as found in either isolated or arranged in pairs, sometimes in chains of four to twenty or more links (Streptococcus; Gr. strepto, a chain, and coccus, a berry).”
1879 – Louis Pasteur (1822-1895) isolated the microorganism from the uteruses and blood of women with puerperal fever and demonstrated that streptococcus was the agent responsible for the disease which had the highest mortality rates for women and newborns at the time.
1884 – Julius Friedrich Rosenbach (1842-1923) examined bacteria isolated from suppurative lesions, and the species was named Streptococcus pyogenes (Gr., pyo, pus, and genes, forming). Initially sub-groups of streptococcus were labelled associated with various diseases such as S.eryespaltis, S. scarlatinae, and S. puerperalis but these were later organised under the single heading of S. pyogenes by Andrewes and Christie in 1932
1889 – Nil Fyodorovich Filatov (1847-1902) described Filatov’s triangle – a diagnostic sign in scarlet fever: marked circumoral pallor that forms a pale triangle around the mouth, contrasting sharply with the flushed cheeks and forehead.
1900 – Clement Dukes numbered the paediatric exanthems to differentiate the variably described and inaccurately labelled rashes of childhood. He divided them based on clinical presentation into: rubeola (first), scarlet fever (second), rubella (third), and Filatov-Dukes (fourth).
1910 – Romanian physician Constantin Chessec Pastia (1883–1926) described Pastia’s lines: linear petechial rash in flexural creases, aiding diagnosis when rash is resolving:
…an intense, continuous linear exanthem localized in the skin folds of the anterior aspect of the elbow. It is of a deep rose color becoming darker in time and after several days even ecchymotic. The lines vary in number from 2 to 4 usually and the skin between these lines presents the rash the same as on the rest of the body.
This sign is given great diagnostic importance in those cases where the eruption is not quite typical and also in retrospective diagnosis, i.e, when the rash has disappeared but the sign remains.
1915 – Frederick Twort (1877-1950), an English bacteriologist, discovered agents that he termed ‘filter-passing viruses‘ which required bacteria for growth. Félix d’Hérelle (1873-1949), a French-Canadian microbiologist, first applied the name ‘bacteriophage‘ (from bacteria and the Greek word phagein, ‘to devour’) to a phage that was able to kill a number of pathogenic bacteria, including streptococci.
1923 – George and Gladys Dick confirmed the role of streptococcal toxin in scarlet fever, developed:
- The Dick test to measure susceptibility
- A vaccine protocol using isolated toxin and antitoxin
1926 – Cantacuzène and Bonciu linked scarlet fever and bacteriophages
1928 – Rebecca Lancefield (1895-1981) classified organisms into various serological groups and identified the group A streptococcus (GAS), Streptococcus pyogenes, as the organism responsible for most of the haemolytic streptococcal infections in humans
1940 – Alexander Fleming (1928), then Howard Florey et al., showed penicillin effective against GAS, revolutionising treatment. “Penicillin as a chemotherapeutic agent“
1964 – Zabriskie showed GAS could acquire toxin genes via bacteriophage T12, converting non-toxigenic strains into erythrogenic toxin producers.
1984 – Independently, Weeks and Ferretti and Johnson and Schlievert, demonstrated that T12 phage contained the structural gene for the streptococcal pyrogenic exotoxin A (speA). In subsequent experiments, the speA containing T12 bacteriophage was shown to integrate into a gene that encodes a serine tRNA in the host chromosome.
1998 – Susan Swedo et al first described the PANDAS hypothesis based on observations in clinical case studies at the US National Institute of Mental Health. In subsequent clinical trials where children appeared to have dramatic and sudden OCD exacerbations and tic disorders following infection.
Associated Persons
- Giovanni Filippo Ingrassias (1510-1580) – first to clearly differentiate scarlet fever from measles and chickenpox
- Daniel Sennert (1572-1637) – classical features of scarlet fever including desquamation and post-infectious oedema
- Michael Döring (1582-1644) – detailed early clinical accounts of scarlet fever during a 1625 Polish epidemic
- Thomas Sydenham (1624-1689) – popularised the term scarlatina and distinguished it from measles
- Pierre-Fidèle Bretonneau (1778-1862) – separated scarlet fever from diphtheria, introducing the term diphthérite
- Nil Fyodorovich Filatov (1847-1902) – Described Filatov mask / triangle
- Clement Dukes (1845-1925) – proposed the numbered classification of paediatric exanthems, listing scarlet fever second
- Constantin Chessec Pastia (1883–1926) – Pastia’s lines, aiding diagnosis of resolving or atypical scarlet fever
- Frederick Twort (1877-1950) – discovered bacteriophages, laying groundwork for understanding streptococcal lysogenic conversion
- Félix d’Hérelle (1873-1949) – coined the term bacteriophage, instrumental in linking phages to streptococcal toxin production
- Rebecca Lancefield (1895-1981) – Defined Group A Streptococcus pyogenes, the key pathogen in scarlet fever.
References
Historical references
- Ioannis Philippi Ingrassiae. De tumoribus praeter naturam, tomus primus 1553: 194-195
- Danielis Sennerti. De febre maligna cum variolis & morbillis. In: Epitome librorum de febribus. 1635; Liber IV, Cap XII: 212-215
- Sydenham T. ‘Febre Scarlatina‘ In: rocessus integri in morbis ferè omnibus curandis. 1693
Eponymous term review
- Bretonneau P. Des inflammations spéciales du tissu muqueux, et en particulier de la diphtérite, ou inflammation pelliculaire, connue sous le nom de croup, d’angine maligne, d’angine gangréneuse, etc.. [Condensed version: “Extrait du traité de la diphthérite, angine maligne, ou croup épidémique” Archives générales de médecine, 1826; 11: 219-254.
- Billroth T. Untersuchungen über die vegetationsformen von coccobacteria septica und den antheil, welchen sie an der entstehung und verbreitung der accidentellen wundkrankheiten haben. Berlin: G. Reimer. 1874
- Rosenbach FJ. Mikro-organismen bei den Wund-Infections-Krankheiten des Menschen, 1884.
- Pastia CC. La Tribune médicale, 1910; 46: 726
- Taubles GH. Diagnostic value of Pastia’s sign in scarlet fever. Cal State J Med. 1912 Jul;10(7):305-6.
- Twort FW. An investigation on the nature of ultra-microscopic viruses. Lancet 1915; 186(4814): 1241-1243
- d’Herelle F. Sur un microbe invisible antagoniste des bacilles dysentériques. Académie des Sciences, 1915; 373-375.
- d’Herelle F. On an invisible microbe antagonistic to dysentery bacilli. Comptes Rendus Academie des Sciences 1917; 165: 373-375
- Cantacuzène J, Bonciu O. Modifications subies par des streptocoques d’origine non scarlatineuse au contact de produits scarlatineux filtrès. Comptes rendus de l’Académie des Sciences. 1926; 182: 1185–1187.
- Rolleston JD. The history of scarlet fever. Br Med J. 1928 nov 24;2(3542):926-9.
- Andrewes FW, Christie EM. The haemolytic streptococci: their grouping by agglutination. London: H.M. Stationery Office, 1932
- Ruhräh J. Michael Doering ?-1644. American journal of diseases of children, 1933; 46(5.1): 1098.
- Ruhräh J. Giovanni Filippo Ingrassias 1510-1580. Archives of Pediatrics & Adolescent Medicine, 1933; 45(2): 372.
- Ruhräh J. Daniel Sennert 1572-1637. Archives of Pediatrics & Adolescent Medicine, 1933; 46(6): 1393.
- Rolleston JD. Scarlet fever. In: The history of the acute exanthemata: the Fitzpatrick lectures for 1935 & 1936
- Zabriskie JB. The role of temperate bacteriophage in the production of erythrogenic toxin by group a streptococci. J Exp Med. 1964;119(5):761-80.
- Weeks CR, Ferretti JJ. The gene for type A streptococcal exotoxin (erythrogenic toxin) is located in bacteriophage T12. Infect Immun. 1984 Nov;46(2):531-6.
- Johnson LP, Schlievert PM. Group A streptococcal phage T12 carries the structural gene for pyrogenic exotoxin type A. Mol Gen Genet. 1984;194(1-2):52-6.
- Weeks CR, Ferretti JJ. Nucleotide sequence of the type A streptococcal exotoxin (erythrogenic toxin) gene from Streptococcus pyogenes bacteriophage T12. Infect Immun. 1986 Apr;52(1):144-50.
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J, Dubbert BK. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998 Feb;155(2):264-71.
- Weir E, Main C. Invasive group A streptococcal infections, CMAJ Jul 2006; 175(1): 32
- Steer AC, Smeesters PR, Curtis N. Streptococcal Serology: Secrets for the Specialist. Pediatr Infect Dis J. 2015 Nov;34(11):1250-2.
- Shapiro L. The numbered diseases: first through sixth. JAMA. 1965 Nov 8;194(6):680.
eponymictionary
the names behind the name

[…] them based on clinical presentation into: measles (first), scarlet fever (second), rubella (third), and Filatov-Dukes (fourth). In 1905, Léon Cheinisse […]