Fungi and Anti-fungal Agents
OVERVIEW
Fungi are a kingdom of eukaryotic organisms distinct from plants and animals that are characterised by thick carbohydrate cellular walls containing glucans and chitin.
- There are over 100,000 known species of fungi (probably more than a million that are unknown), with over 300 known species that are pathogenic (either obligatory or opportunistic)
- Candida and the dermatophytes transmit human-to-human (or animal-to-human), others are acquired from the environment (e.g. by inhalation, penetrating injury, or invasive devices)
- Mycosis is the term for fungal disease – serious mycoses typically occur in the context of immunosuppression and hospital-acquired infection.
- Mycosis can be treated by a variety of different anti-fungal agents
CLASSIFICATION OF FUNGI BY GROWTH FORM
Yeasts
- Yeasts are microscopic eukaryotic, single-celled microorganisms
- Yeast reproduce by budding, and pseudohyphae form when buds remain attached
- May survive and multiply within host phagocytic cells as well as extracellularly
- E.g. Candida, Cryptococcus, Pneumocystis
Molds
- microscopic multicellular organisms typically arranged into filaments called hyphae
- A mass of hyphae is termed a micellium
- Spores, which may cause disease through inhalation, are released from sacs known as sporangia and are produced by asexual reproduction
- E.g. Aspergillus, Zygomycetes (Mucor, Rhizopus), Other (Scedosporium, Fusarium)
Dimorphic fungi exist in both yeast and mold forms (e.g. Candida can form hyphae in the body)
Mushrooms
- the macroscopic fleshy, spore-bearing fruiting body of a fungus, typically produced above ground on soil or on its food source.
- Not usually pathogenic, but may be toxic!
CLASSIFICATION OF FUNGI BY TYPE OF INFECTION
- Superficial mycoses (e.g. oral thrush, tinea) – fungi grow on body surfaces
- subcutaneous infections (e.g. mycetoma, sporotrichosis) – involve nails or deeper layers of the skin
- Systemic or deep mycoses – involve internal body organs and include:
- endemic mycoses (e.g. histoplasmosis, blastomycosis, coccidioidomycosis) – infect immunocompetent people
- opportunistic mycoses (e.g. systemic candidiasis, aspergillosis, Pneumocystis) – infect immunocompromised people (most important category in critical care); organisms may be part of the normal body flora
- Diseases caused by free living fungi that produce toxins (e.g. aflatoxin) or spores (e.g. acute bronchopulmonary aspergillosis (ABPA))
ANTI-FUNGAL AGENTS
Anti-fungals
- Neutrophils play a major role in the host response to mycosis
- The distinctive nature of the fungal cell wall and the predominance of ergosterol – rather than cholesterol – in fungal cell membranes are important targets for anti-fungal action
- Limitations of anti-fungals include:
- Fungal selectivity of anti-microbial agents is more difficult to achieve than for antimicrobials targeting prokaryotes
- Stability, solubility, and toxicity of some agents (especially amphotericin B)
- Resistance to azoles is becoming more widespread and resistance to flucytosine means it should not be used as monotherapy
Mechanisms of resistance against anti-fungal agents include:
- Enzyme modification (e.g. destruction of drug)
- Target modification
- Reduced permeability/ entry of drug into pathogen
- Active efflux pumps
- Failure to activate antifungal agents
Brief overview of common therapies for fungal infections that are important in critical care:
| Aspergillosis | voriconazole Liposomal amphotericin B |
| Candidiasis | Superficial Clotrimazole Miconazole Nystatin Fluconazole Systemic Echinocandins: Caspofungin, micafungin, anidulafungin Liposomal amphotericin Ocular or CNS infection Liposomal amphotericin and flucytosine |
| Cryptococcosis | Liposomal amphotericin B and flucytosine |
| Histoplasmosis | Liposomal amphotericin B then itraconazole |
| Mucormycosis | Liposomal amphotericin B Surgery often required |
| Pneumocystis pneumonia | co-trimoxazole Pentamidine, dapsone (second line agents) |
CLASSIFICATION OF ANTIFUNGAL AGENTS
There are five main classes of antifungal agents.
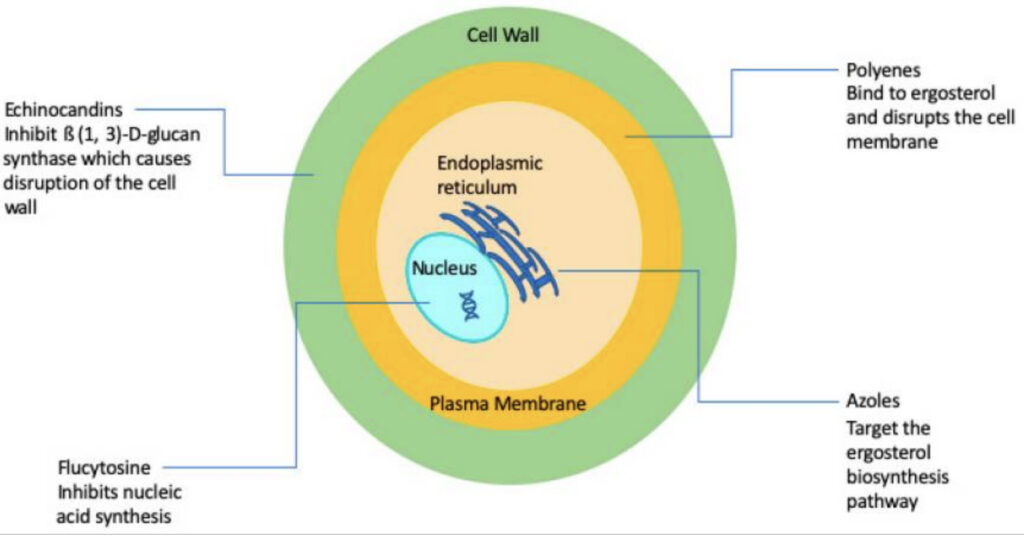
- Polyenes – selectively bind ergosterol
- e.g. Amphotericin B, Nystatin
- Azoles – prevent the synthesis of ergosterol from lanosterol by inhibiting lanosterol-C14-α-demethylase
- Imidazoles – e.g. clotrimazole, miconazole, ketoconazole
- Triazoles
- First generation – e.g. fluconazole, itraconazole
- Second generation (extended spectrum) – e.g. voriconazole, posaconazole
- Thiazoles – e.g. abafungin
- Allylamines – inhibit squalene epoxidase and prevent ergosterol synthesis
- e.g. Amorolfin, Butenafine, Naftifine, Terbinafine
- Echinocandins – inhibit glucan synthesis in the fungal cell wall
- e.g. Anidulafungin, Caspofungin, Micafungin
- Others –
- e.g. Griseofulvin (a microtubule inhibitor), Flucytosine (5FC) (inhibits nuceic acid synthesis), Pentamidine
TISSUE PENETRATION OF ANTI-FUNGALS
Anti-fungal agents must penetrate tissues that are the site of infection and achieve concentrations capable fo eliminating the pathogen to be effective (Felton et al, 2014).
- small polar agents with low protein binding (e.g., fluconazole and 5FC) distribute more evenly and into a wider range of tissues than the larger, more lipophilic (itraconazole) or amphipathic (e.g., amphotericin B and echinocandins) agents.
- more lipophilic or amphipathic agents may have longer residence times within tissues and may accumulate to concentrations that exceed those in the plasma.
- agents with relatively low molecular weights, such as fluconazole, 5FC, and voriconazole, penetrate more readily into tissue beds.
- formulations may have a significant impact on serum and tissue pharmacokinetics.
- the measurable concentration of a drug within a tissue may not be an indication of its biological activity in that compartment.
- marked differences in tissue distribution can occur within a single drug class even with closely related structures (e.g., the triazoles).
References and Links
FOAM and web resources
- LITFL
- CCC — Aspergillosis
- CCC — Candidiasis
- CCC — Cryptococcosis
- CCC — Mushroom toxicity
- CCC — Pneumocystis jiroveci
- CCC — Rhinocerebral Mucormycosis
- CCC — Amphotericin B (conventional and liposomal)
- CCC — Caspofungin
- CCC — Fluconazole
- CCC — Voriconazole
- EMCrit Internet Book of Critical Care — Anti-fungals (2020)
- ICN Podcast — 69. Janin on Fungal Infections (2012)
- Maryland CC Project — Fungal Invasion of the ICU: A catastrophe waiting to happen (2014)
Journal articles
- Brunton, L., Hilal-Dandan, R., Knollman, B. (2017). Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition. United States: McGraw-Hill Education. Ch 61.
- Chatelon J, Cortegiani A, Hammad E, Cassir N, Leone M. Choosing the Right Antifungal Agent in ICU Patients. Adv Ther. 2019 Dec;36(12):3308-3320. doi: 10.1007/s12325-019-01115-0. Epub 2019 Oct 15. PMID: 31617055; PMCID: PMC6860507.
- Dockrell, H., Goering, R., Chiodini, P. L., Zuckerman, M. (2018). Mims’ Medical Microbiology and Immunology. United Kingdom: Elsevier. Ch 4, 34.
- Enoch DA, Ludlam HA, Brown NM. Invasive fungal infections: a review of epidemiology and management options. J Med Microbiol. 2006 Jul;55(Pt 7):809-18. PMID: 16772406.
- Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev. 2014 Jan;27(1):68-88. doi: 10.1128/CMR.00046-13. PMID: 24396137; PMCID: PMC3910906.
- Mourad A, Perfect JR. Tolerability profile of the current antifungal armoury. J Antimicrob Chemother. 2018 Jan 1;73(suppl_1):i26-i32. doi: 10.1093/jac/dkx446. PMID: 29304209; PMCID: PMC6636388.
- Van Thiel DH, George M, Moore CM. Fungal infections: their diagnosis and treatment in transplant recipients. Int J Hepatol. 2012;2012:106923. PMC3433127.
- Wall G, Lopez-Ribot JL. Current Antimycotics, New Prospects, and Future Approaches to Antifungal Therapy. Antibiotics (Basel). 2020 Jul 25;9(8):445. doi: 10.3390/antibiotics9080445. PMID: 32722455; PMCID: PMC7460292.

Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC
