Paracetamol toxicity
Guest Post Dr Angela Chiew – Australian Clinical Toxicologist and Emergency Staff Specialist
The majority of paracetamol overdoses/ poisoning are straight forward, and standard treatment protocols can be applied. Most patients will present following a single immediate-release paracetamol ingestion of < 20 g.
The difficultly in the management of paracetamol poisoning is recognising those situations where treatment may differ.
Dr Angela Chiew 2020
That is overdose scenarios where the paracetamol nomogram can not be utilised or where increased or prolonged doses of acetylcysteine are required. The other difficulty with the management of paracetamol poisoning is that guidelines vary worldwide from what is defined as a toxic ingestion, to what nomogram line should be utilised and even how to administer acetylcysteine. Hence your local guidelines should be consulted as they may differ from the recommendations made in this review. This overview is based on the Australian and New Zealand Guidelines which is one of the few paracetamol guidelines published and freely available:
Summary Statement: https://www.mja.com.au/system/files/issues/212_04/mja250428.pdf
Full Guidelines: https://www.mja.com.au/sites/default/files/issues/212_04/mja250428-sup-0001-Supinfo.pdf
Key Points to remember:
- Survival from a paracetamol overdose is generally considered to be 100% in cases receiving NAC within 8 hours of exposure. Efficacy declines after this point.
- The threshold for potential paracetamol-induced hepatic injury in adults is >10g or >200 mg/kg (whichever is less) within 24 hours. So, 10g is the toxic dose for all those heavier than 50kg.
- There is no standard definition of “massive” paracetamol ingestion but those with an initial paracetamol concentration greater than double the 150mg at 4 h nomogram line are at higher risk of acute liver injury and require higher doses of acetylcysteine.
- Nomogram limitations:
- The nomogram has only be validated for acute (single) immediate-release paracetamol ingestions where an accurate time of ingestion is know.
- Always check the units – mg/L vs µmol/L.
- Extrapolation beyond 15-24 hours and modified release preparations are not validated.
- When in doubt, conservative management is recommended, particular if the patient co-ingested substances that might prolong absorption (i.e. anticholinergic agents that decrease gastric emptying) and is close to the line. In these cases, start or continue NAC until the risk assessment can be refined using further biochemical parameters and paracetamol concentrations.
- Screening and testing for paracetamol in the first 24 hours is necessary as patients are asymptomatic with toxic doses. Beyond 24 hours, symptoms should be evident (nausea, vomiting, abdominal pain or encephalopathy). Equally do not discharge the symptomatic patient, you may have mis-timed the point of ingestion or the patient may have lied to you (about time or co-ingestants).
Toxic Mechanism:
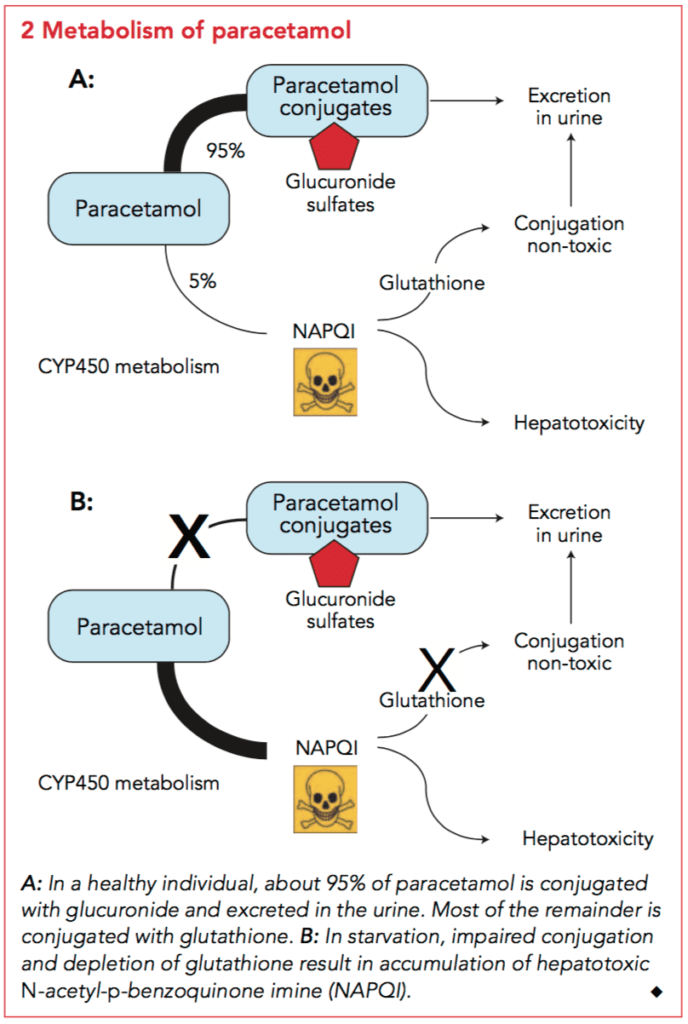
The main toxicity following paracetamol poisoning is acute liver injury which results from the formation of a toxic metabolite of paracetamol, N-acetyl-p-benzoquinone imine (NAPQI). In adults taking therapeutic doses, paracetamol is metabolised into two major non-toxic metabolites – sulphate and glucuronide conjugates – which account for 30% and 55% of paracetamol metabolism. NAPQI, is formed in small amounts following a therapeutic dose of paracetamol. It is a highly reactive toxic metabolite formed by cytochrome P450 2E1 and is responsible for the hepatocellular injury that occurs with paracetamol toxicity. The small amounts of NAPQI produced after therapeutic doses are detoxified by irreversible glutathione-dependent conjugation reactions to two non-toxic metabolites. In overdose, the increased formation of NAPQI depletes glutathione and once glutathione is depleted by more than two-thirds it covalently binds to critical cellular proteins. It is hypothesised that this results in loss of activity of critical proteins and eventually hepatic cell death.
To summarise, NAPQI is bad, in therapeutic doses glutathione can make in non-toxic. In overdose the glutathione mechanism is overwhelmed. Hence NAC works as a glutathione donor preventing NAPQI accumulation
Paracetamol metabolism diagram
Toxicokinetics:
- Well absorbed from the small intestine
- Peak concentration 1-2 hours post ingestion (30 mins for liquid)
- Small volume of distribution 0.9 L/kg
- Hepatic metabolism and renal excretion as shown above.
- Modified release in Australia 665mg of paracetamol contains 69% slow release and 31% immediate release paracetamol. It has a prolonged absorption phase with peak concentration occurring within 2-3 hours but maybe delayed up to 20 hours in overdose.
Resuscitation:
- Rarely required: in massive overdoses (high levels > 800mg/L can cause coma and a lactic acidosis) or delayed presentation with hepatic failure.
- Attention to airway, breathing and circulation.
- Correct hypoglycaemia.
Risk Assessment:
Risk assessment to guide management involves an accurate history and investigations:
- Dose
- Formulation ingested – immediate or modified release
- Acute vs staggered vs repeated supratherapeutic ingestion
- Time of ingestion
- Symptoms: abdominal pain, nausea, vomiting
Investigations:
- All patients:
- Serum paracetamol concentration
- ALT/AST
- Those patients with acute liver injury also require:
- Venous blood gas – pH and lactate
- EUC
- Glucose
- Coags
- Calcium / Magnesium / Phosphate
Paracetamol Nomogram
Limitations of the nomogram:
- Applies only to single acute paracetamol ingestions of immediate-relate
- Does not apply to co-ingestants, modified-release paracetamol ingestions (hence case reports of “line crossers”)
- Not validated before 4 hours or after 16 hours (however extrapolated to 24 hours)
- Each country has different nomograms and units – make sure you are using the correct one and correct scale
Decontamination
- Immediate-release 50g of activated charcoal within 2 hours (up to four hours if >30g of paracetamol)
- Modified-release 50g of activated charcoal within 4 hours (>30g or >500 mg/kg may benefit from multiple doses as absorption may continue up to 24 hours – seek expert advice).
- Activated charcoal dose in children is 1g/kg – max 50g
Antidote
- Intravenous NAC
- See indications in the list of scenarios below
- NAC can mildly elevate the INR to 1.3 – if stable this is not the start of hepatic failure.
- Anaphylactoid reactions occur anywhere between 10-50% and are more likely to occur in patients with lower paracetamol ingestions (NAPQI appears to be protective). Typically this reaction occurs after the first bag of NAC.
- Stop the infusion
- Treat with loratadine 10mg (2.5mg <12kg, 5mg <30kg) PO or promethazine 12.5 mg IV (0.25mg/kg)
- The NAC can then be recommenced once symptoms settle at half rate for 30 minutes and then recommenced as per normal protocol.
- Anaphylactic reaction: It is rare but there are reported cases of a true anaphylactic reaction and the patient should be treated along conventional guidelines.
Disposition
- The majority of paracetamol ingestions can be managed on the ward.
- Rarely patients with an initial paracetamol concentration below the treatment nomogram line develop acute liver injury hence all patients should be advised to return if they develop abdominal pain, nausea or vomiting for further assessment.
- In all patients who receive 20 h of acetylcysteine, 2 h before completion of the infusion check ALT/AST and paracetamol concentration (in those with high initial paracetamol concentrations greater than double the 150mg at 4 h nomogram line OR modified-release ingestions) to determine need for ongoing acetylcysteine.
- In the rare case of massive paracetamol overdose and metabolic dysfunction the patient will require intensive care.
- Patients with hepatotoxicity (AST/ALT >1000 IU/L) and rising INR will require discussion with a clinical toxicology service.
Hepatotoxicity and Subsequent Liver Failure:
- Only a small proportion of patients develop hepatotoxicity (ALT > 1000 U/L); early symptoms include nausea, vomiting, abdominal pain and right upper quadrant tenderness. Of these only a minority will develop fulminant hepatic failure, and most patients recover fully with standard treatments. Typically, in those with paracetamol induced acute liver injury the ALT and AST will rise for 3 – 4 days before recovering.
- Acetylcysteine is continued until the criteria met above.
- Investigations that monitor liver function and guide prognosis should be performed regularly in all patients with hepatotoxicity, including electrolytes, urea, creatinine, liver function tests, INR, blood sugar, phosphate and venous blood gas (looking at the pH and lactate).
It is recommended that a Liver Transplant Unit should be consulted if any of the following criteria are met (also known as the Modified King’s college criteria (see resources for controversies but remember these criteria were developed to allow identification of patients with liver failure, so that expert advice can be sort and are not transplant criteria.):
- INR >3.0 at 48 hours or >4.5 at any time
- Oliguria or creatinine > 200 micromol/L
- Acidosis with pH < 7.3 after resuscitation or arterial lactate > 3 mmol/L
- Systolic hypotension with BP < 80mmHg despite resuscitation
- Hypoglycaemia
- Severe thrombocytopenia
- Encephalopathy of any degree (if discussions are needed with the patient it is best to make these referrals early before the patient becomes too confused).
- or any alteration of consciousness (GCS<15) not associated with sedative co-ingestions.
DO NOT GIVE clotting factors unless bleeding or after discussion with a Liver Transplant Unit.
Recommendations on when to seek further advice from Poisons Information Centre
These are situations where the risk of hepatotoxicity and complications are greater, the optimum advice is potentially changing, and where it may be most useful to seek advice.
- Very large overdoses: immediate release or modified release paracetamol overdoses of ≥ 50 g or 1 g/kg (whichever is less).
- High paracetamol concentration, more than triple the nomogram line.
- Intravenous paracetamol errors/overdoses, as the treatment threshold is lower.
- Patients with hepatotoxicity (i.e. ALT > 1000 IU/L).
- Neonatal paracetamol poisonings
Below are the most common presentations and how to manage them:
Single ingestion < 8 hours (Immediate release)
- The patient is at risk of hepatic injury if >10g or 200mg/kg (whichever is less) of paracetamol has been ingested.
- Give activated charcoal within 2 hours if 10 to 30g ingested or within 4 hours if ≥30g ingested.
- Measure serum paracetamol concentration and ALT/AST > 4 hours post ingestion.
- If paracetamol concentration result can be determined before 8 hours, wait to start acetylcysteine and plot paracetamol concentration against the nomogram. If a paracetamol concentration cannot be determined within 8 hours, start NAC until the results are available.
- In those who require acetylcysteine, 2 hours before completion of infusion check ALT/AST and paracetamol concentration (in those with high initial paracetamol concentrations greater than double the 150mg at 4 h nomogram line) to determine need for ongoing acetylcysteine.
- Acetylcysteine should be continued in those patients who have a persistently high paracetamol concentration > 10 mg/L (66 μmol/L) OR ALT is > 50 U/L and increasing. Note: small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%) are common and do not on their own indicate the need for ongoing acetylcysteine.
Single ingestion 8 – 24 hours
- The patient is at risk of hepatic injury if >10g or 200mg/kg (whichever is less) of paracetamol has been ingested.
- Commence NAC infusion
- Measure paracetamol concentration and ALT/AST levels
- If the paracetamol concentration is under the treatment nomogram line and ALT/AST is <50 IU/L cease acetylcysteine. Otherwise continue and complete 20 hour acetylcysteine infusion.
- In those who require 20 h of acetylcysteine, 2 h before completion of acetylcysteine infusion check ALT/AST and paracetamol concentration (in those with high initial paracetamol concentrations greater than double the 150mg at 4 h nomogram line) to determine need for ongoing acetylcysteine.
- Acetylcysteine should be continued in those patients who have a persistently high paracetamol concentration > 10 mg/L (66 μmol/L) OR ALT is > 50 U/L and increasing. [note: small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%)] are common and do not on their own indicate the need for ongoing acetylcysteine).
Single ingestion > 24 hours
- The patient is at risk of hepatic injury if >10g or 200mg/kg (whichever is less) of paracetamol has been ingested.
- Start acetylcysteine.
- If the paracetamol concentration is > 10mg/L or the ALT/AST is >50 IU/L complete the 20 hour acetylcysteine infusion.
- In those who require 20 h of acetylcysteine, 2 h before completion of acetylcysteine infusion check ALT/AST and paracetamol concentration (in those with high initial paracetamol concentrations greater than double the 150mg at 4 h nomogram line) to determine need for ongoing acetylcysteine.
- Acetylcysteine should be continued in those patients who have a persistently high paracetamol concentration > 10 mg/L (66 μmol/L) OR ALT is > 50 U/L and increasing. [note: small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%)] are common and do not on their own indicate the need for ongoing acetylcysteine).
Massive paracetamol ingestion ≥ 30g or ≥ 500mg/kg or paracetamol concentration double the nomogram line
- Patients who have a paracetamol concentration greater than double the 150mg/L at 4 h nomogram line have an increased risk of hepatotoxicity.
- Based on observational study data it is the current Australian and New Zealand recommendation to increase the dose of acetylcysteine in those with high initial paracetamol concentration from 100 mg/kg to 200 mg/kg over 16 hrs.
- Some patients may require even higher doses of acetylcysteine and patients with an initial paracetamol concentration > triple the nomogram line should be discussed with a clinical toxicologist.

Staggered ingestion < 24 hours
- The patient is at risk of hepatic injury if >10g or 200mg/kg (whichever is less) of paracetamol has been ingested.
- One published strategy here is to treat the patient as if they took all of their tablets at the first (earliest) point in time and treat them along the same strategies as above.
- However, if the first paracetamol concentration was measured within 2 hours of the last ingested paracetamol dose it should be repeated after 2 hours to ensure there is no ongoing absorption. If either concentration is above the nomogram line (using time from the earliest ingestion), start/continue treatment with acetylcysteine.
Example 1: Staggered overdose < 8 hours e.g. 4g at 1pm, 4g at 2pm and 4g at 3pm and its now 4pm use the <8hr guideline above and time anchor the paracetamol concentration to 1pm. This allows for the worst-case scenario. If you time anchor to 3pm you may have a lower concentration and underestimate the overdose. The paracetamol concentration should be repeated at 6 pm (2 h after the first concentration) and time anchor concentration to 1pm.
Example 2: If the patient has a staggered overdose throughout the day over 8 hours, e.g. 4g at 0800 then 4g at 1200 and 4g at 1700, treat them as per the 8-24 hr guideline above. Start acetylcysteine and time anchor the paracetamol concentration to 0800. Repeat the paracetamol concentration if the first paracetamol concentration was measured within 2 hours of the last ingested paracetamol dose.
Staggered ingestion over 24 hours
- Treat as per an acute ingestion.
- The patient is at risk of hepatic injury if >10g or 200mg/kg (whichever is less) of paracetamol has been ingested.
- Start acetylcysteine.
- If the paracetamol concentration is > 10mg/L or the ALT/AST is >50 IU/L complete the 20 hour acetylcysteine infusion.
- In those who require 20 h of acetylcysteine, 2 h before completion of acetylcysteine infusion check ALT/AST and paracetamol concentration (in those with high initial paracetamol concentrations greater than double the 150mg at 4 h nomogram line) to determine need for ongoing acetylcysteine.
- Acetylcysteine should be continued in those patients who have a persistently high paracetamol concentration > 10 mg/L (66 μmol/L) OR ALT is > 50 U/L and increasing. [note: small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%)] are common and do not on their own indicate the need for ongoing acetylcysteine).
Modified release ingestion
In Australia, New Zealand and Europe modified-release paracetamol contains 69% modified-release and 31% immediate-release paracetamol in a 665 mg tablet. There are different formulations of modified-release paracetamol and the product available in the USA is a 50:50 mix. Management of modified-release paracetamol in Australia and Europe was previously based on treatment of immediate-release paracetamol ingestion. However, evidence from studies and case series from both Australia and Europe have shown that this approach appears inadequate.
Importantly, the nomogram should not be used to assess need for treatment of potentially toxic modified-release ingestions. Paracetamol concentrations are useful to guide further management such as acetylcysteine dosage (e.g. the need for increased or prolonged treatment) and need for further decontamination (e.g. further doses of activated charcoal if paracetamol concentrations remain unchanged or rise).
Modified-release ingestion ≥ 10 g or 200 mg/kg (whichever is less)
- Offer activated charcoal up to 4 hours post-ingestion. For massive (≥ 30 g) modified-release paracetamol overdoses, absorption may continue up to 24 hours post-ingestion; patients may benefit from activated charcoal beyond 4 hours.
- Immediately commence acetylcysteine, all to receive a full 20-hour course of acetylcysteine regardless of their serum paracetamol concentration(s).
- All patients who ingest ≥ 30 g or ≥ 500 mg/kg of modified release paracetamol or have a paracetamol concentration greater than double the nomogram line should receive an increased dose of acetylcysteine (that is 200 mg/kg over 16 hours).
- 2 hours before completion of acetylcysteine infusion check ALT/AST and paracetamol concentration to determine need for ongoing acetylcysteine.
- Acetylcysteine should be continued in those patients who have a persistently high paracetamol concentration > 10 mg/L (66 μmol/L) OR ALT is > 50 U/L and increasing. [note: small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%)] are common and do not on their own indicate the need for ongoing acetylcysteine).
Modified-release ingestion < 10 g and < 200 mg/kg.
- Two serum paracetamol concentrations 4 hours apart, starting at least 4 h post-ingestion.
- If either is above the nomogram line, a standard course of acetylcysteine should be given.
Repeated Supratherapeutic ingestion (RSTI)
- Patients who ingest excessive paracetamol for a therapeutic purpose (e.g. pain, viral illness) or ingest therapeutic doses of paracetamol and have symptoms of acute liver injury (e.g. abdominal pain, nausea and vomiting) are managed as RSTI
- The paracetamol nomogram is not useful
- Management of RSTI varies worldwide from the definition of a toxic ingestion to treatment with acetylcysteine.
Management RSTI (Australian and New Zealand guidelines)
A risk assessment needs to be performed history of ingestion and those at measure a paracetamol concentration and ALT/AST.
Patients at risk:
- ≥ 10 g or ≥ 200 mg/kg (whichever is less) over a single 24 h period. Child = >200mg/kg
OR
- ≥ 12 g or ≥ 300 mg/kg (whichever is less) over a single 48 h period. Child = >150mg/kg per 24 hours period for the last 48 hours.
OR
- ≥ a daily therapeutic dose^ per day for more than 48 h in those who also have abdominal pain or nausea or vomiting.
Note: ^ Therapeutic daily dose of paracetamol in adults is a total dose of 60 mg/kg over 24 h and up to a maximum dose of 4 g/day. For paediatric dosage refer to local guidelines.
If above criteria are met, then measure ALT/AST and paracetamol concentration:
- If paracetamol concentration is >20 mg/L (>120 micromol/L) or the ALT or AST is > 50 IU/L start acetylcysteine.
- Repeat ALT/AST and paracetamol concentration 8 hours after the initial sampling. Those with significant acute liver injury secondary to paracetamol will have a very high and/or rapidly rising ALT. Those with a paracetamol concentration > 10mg/L or ALT that is increasing should complete the 20 h course of acetylcysteine
- Small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%) are common and do not on their own indicate the need for ongoing acetylcysteine.
- Those with an ALT or AST > 1000 U/L should receive at least a full 20 h course of acetylcysteine.
- In those that receive 20 h of acetylcysteine, 2 h before completion of acetylcysteine infusion check ALT/AST to determine need for ongoing acetylcysteine.
- Acetylcysteine should be continued in those patients with an ALT > 50 U/L and is increasing.
Unknown time of ingestion
- Either the patient refuses to tell you, you have doubts about the timing or they are in a coma and paracetamol is detectable, start acetylcysteine.
- Acetylcysteine can be ceased when either a history is obtained or if hepatic transaminases are found to be < 50 U/L at the end of a 20-hour NAC infusion
Cessation of Acetylcysteine
In all those patients who require acetylcysteine beyond 20 hours. Acetylcysteine can be ceased if all the following criteria have been met:
- ALT or AST are decreasing
- INR < 2.0
- Patient clinically well
AND
For modified-release ingestions and those with an initial paracetamol concentration greater than double the nomogram line: paracetamol concentration < 10 mg/L (66 μmol/L)
Paediatric (<6 years) liquid paracetamol ingestion:
Paediatric patients (children <6 years) are thought to be less susceptible to paracetamol toxicity. Furthermore they usually present with accidental ingestions. They have rapid absorption and an earlier time to peak paracetamol concentration.
- Activated charcoal is not indicated in this group
- Ingestions over 200mg/kg can have a 2 hour paracetamol concentration.
- If the 2 to 4 hour concentration is below 150 mg/L (1000 μmol/L) acetylcysteine is not required. If it is above this level then repeat a paracetamol concentration at 4 hours post ingestion. If at four hours the level is above 150 mg/L (1000 μmol/L) then start acetylcysteine.
- The above only applies for the well child under the age of 6 with liquid paracetamol. In all other cases a 4 hour concentration should be performed.
- Those children who present 4 hours post-ingestion or in children older than 6 years of age, treatment is as per the adult paracetamol exposure guidelines.
References and Additional Resources:
Additional Resources:
- Toxicology conundrum 045 – Paracetamol-Induced Hepatotoxicity
- Toxicology conundrum 001 – Paracetamol…too much, too often?
- CCC – Acute paracetamol toxicity
- CCC – Liver transplant post paracetamol
References:
- Cairney DG, Beckwith HK, Al-Hourani K, Eddleston M, Bateman DN, Dear JW. Plasma paracetamol concentration at hospital presentation has a dose-dependent relationship with liver injury despite prompt treatment with intravenous acetylcysteine. Clin Toxicol (Phila) 2016; 54(5): 405-10.
- Chiew AL, Fountain JS, Graudins A et al. Summary statement: new guidelines for the management of paracetamol poisoning in Australia and New Zealand. MJA 2015; 203(5):215-218
- Chiew AL, Isbister GK, Duffull SB, Buckley NA. Evidence for the changing regimens of acetylcysteine. British journal of clinical pharmacology. 2016;81(3):471-81.
- Chiew AL, Isbister GK, Kirby KA, Page CB, Chan BSH, Buckley NA. Massive paracetamol overdose: an observational study of the effect of activated charcoal and increased acetylcysteine dose (ATOM-2). Clin Toxicol (Phila). 2017;55(10):1055-65.
- Chiew AL, Isbister GK, Page CB, Kirby KA, Chan BSH, Buckley NA. Modified release paracetamol overdose: a prospective observational study (ATOM-3). Clin Toxicol (Phila). 2018;56(9):810-9.
- Chiew AL, Reith D, Pomerleau A, Wong A, Isoardi KZ, Soderstrom J, et al. Updated guidelines for the management of paracetamol poisoning in Australia and New Zealand. The Medical journal of Australia. 2020;212(4):175 – 83.
- Daly FS, Fountain JS, Murray L et al. Guidelines for the management of paracetamol poisoning in Australia and New Zealand – explanation and elaboration. A consensus statement from toxicologists consulting to the Australasian Poisons Information Centres. Medical Journal of Australia 2008; 188:296-301.
- Dart RC, Erdman AR, Olson KR et al. Acetaminophen poisoning: an evidence-based consensus guideline for out-of-hospital management. Clinical Toxicology 2006; 44:1-18.
- Daly FFS, O’Malley GF, Heard K et al. Prospective evaluation of repeated supratherapeutic acetaminophen (paracetamol) ingestion. Annals of Emergency Medicine 2004; 44(4):393-398.
- Egan H, Isbister GK, Robinson J, Downes M, Chan BS, Vecellio E, et al. Retrospective evaluation of repeated supratherapeutic ingestion (RSTI) of paracetamol (dagger). Clin Toxicol (Phila). 2019;57(8):703-11.
- Hendrickson, R.G., What is the most appropriate dose of N-acetylcysteine after massive acetaminophen overdose? Clinical Toxicology, 2019. 57(8): p. 686-691.
- Marks DJB, Dargan PI, Archer JRH, et al. Outcomes from massive paracetamol overdose: a retrospective observational study. Br J Clin Pharmacol 2017; 83(6): 1263-72.
- Murray L et al. Toxicology Handbook 3rd Edition. Elsevier Australia 2015. ISBN 9780729542241
- O’Grady JG. Acute liver failure. Postgraduate Medical Journal 2005; 81:148-154.
- Pettie, J.M., et al., Safety and Efficacy of the SNAP 12-hour Acetylcysteine Regimen for the Treatment of Paracetamol Overdose. EClinicalMedicine, 2019. 11: p. 11-17.
- Prescott LF. Paracetamol (acetaminophen). A critical bibliographic review. 2nd edn. Taylor and Francis, London 2001.
- Prescott LF, Illingworth RN, Critchley JA. Intravenous N-acetylcysteine: the treatment of choice for paracetamol poisoning. British Medical Journal 1979; 2:1097.
- Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. Journal of Toxicology-Clinical Toxicology 2002; 40:3-20.
- Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics 1975; 55:871-876.
- Schiødt FV, Bondesen S, Tygstrup N et al. Prediction of hepatic encephalopathy in paracetamol overdose: a prospective and validated study. Scandinavian Journal of Gastroenterology 1999; 34(7):723-8
- Silvalotti MCA, Yarema MC, Juurlink DN et al. A risk quantification instrument for acute acetaminophen overdose patient treatment with N-acetyl cysteine. Annals of Emergency Medicine 2005; 46(3):263-271.
- Wong, A. and A. Graudins, Simplification of the standard three-bag intravenous acetylcysteine regimen for paracetamol poisoning results in a lower incidence of adverse drug reactions. Clin Toxicol (Phila) 2016. 54(2): p. 115-119.
Toxicology Library
DRUGS and TOXICANTS
Dr Neil Long BMBS FACEM FRCEM FRCPC. Emergency Physician at Kelowna hospital, British Columbia. Loves the misery of alpine climbing and working in austere environments (namely tertiary trauma centres). Supporter of FOAMed, lifelong education and trying to find that elusive peak performance.