OMI: Replacing the STEMI misnomer
Under the current STEMI paradigm, 25-30% of NSTEMI patients are found to have total occlusion on delayed cardiac catheterisation. Using expert ECG interpretation instead of strict STEMI criteria, cardiologists are able to successfully reclassify 28% of NSTEMI patients as having acute coronary occlusion responsive to immediate reperfusion therapy, halving short- and long-term mortality
The DIFOCCULT Study, Int J Cardiol Heart Vasc. 2020 Oct; 30
In 2018, Meyers, Weingart and Smith introduced us to the concept of Occlusion Myocardial Infarction (OMI) through the OMI Manifesto. Since this, there has been gaining awareness that ST elevation on ECG is most likely an unreliable tool for detecting patients that will benefit from PCI, and that a shift is required to a more reliable paradigm for detecting acute coronary occlusion.
We utilise “STEMI criteria” in everyday practice but conveniently ignore the 2.5mm STE on the ECG of a 30-year-old male with benign sounding chest pain, whilst scrutinising over the trace STE in an 80-year-old female with ischaemic-sounding chest pain. The term “STEMI equivalent” is already in our vocabulary, but really it is referring to patients with clinical and ECG features concerning for acute coronary occlusion that would benefit from immediate percutaneous coronary intervention (PCI).
Australian guidelines recommend PCI for a number of patient groups with NSTEACS, however with differing time interval guidelines, ranging from 2 to 72 hours, and arbitrary criteria. These include a recommendation for high risk groups with dynamic ST segment or T waves changes to receive intervention within 24 hours. Outside of this there is no specific recommendation as to which ECG findings are indicative of more immediate management. Those with recurrent symptoms are suggested for intervention within 72 hours by which time reversal of any acute occlusion is going to be of little benefit to infarcted tissue.
ST depression in 2 or more precordial leads (V1-4) may indicate transmural posterior injury; multilead ST depression with coexistent ST elevation in lead aVR has been described in patients with left main or proximal LAD occlusion. Hyperacute T-wave changes may be observed in the early phase of STEMI, before the development of ST elevation.
2013 ACCF/AHA STEMI Guideline
The ACCF/AHA guidelines recognise the significance of other ECG patterns in predicting occlusion, however they are yet to be incorporated into guidelines and remain “patterns to be aware of” for the practicing clinician. The publication recognises that baseline ECG abnormalities such as LV hypertrophy may obscure ECG interpretation, but does not offer a recommendation on ways to overcome this.
What is an OMI?
Occlusion Myocardial Infarction (OMI): A branch of the ACS algorithm representing near or total occlusion with insufficient collateral circulation causing active infarction
Non-Occlusion Myocardial Infarction (NOMI): No occlusion, or sufficient collateral circulation to avoid active infarction
Meyers HP, Smith SW. 2020 EM News
- Patients with Occlusion Myocardial Infarction (OMI) are those that benefit from emergent reperfusion therapy, and in which the benefits outweigh the risks of this invasive procedure
- There are a number of ECG patterns that can represent occlusion, these and the clinical features can be used to guide diagnosis and raise suspicion for an OMI. Some of these such as Sgarbossa criteria we may already be familiar with
- There is no single criterion and the clinical context is an important factor. Both European and American guidelines recommend that any patient with ongoing ischaemic chest pain be considered for immediate angiography
- The STEMI/NSTEMI paradigm is not a reliable tool for diagnosing occlusion MI
What’s wrong with the STEMI label?
Patients with acute occlusion not meeting STEMI criteria may be an underserved, underidentified subgroup of ACS patients who would benefit from emergent intervention, whereby classification of AMI by occlusion vs. no occlusion may be more appropriate than classification by ST elevation on the ECG (sic).
Meyers et al 2020
- Clinicians don’t miss myocardial infarction under the STEMI/NSTEMI paradigm, they miss acute occlusions responsive to reperfusion therapy
- Under the STEMI/NSTEMI paradigm, up to 30% of patients we classify as NSTEMI are consistently found to have missed acute coronary occlusion. Although these patients often end up receiving intervention at a later stage (24-72 hours) into admission, it is usually too late to salvage ischaemic or infarcted tissue and they are exposed to significant increases in morbidity and mortality
- A number of patients with benign ST elevation undergo unnecessary catheterisation +/- reperfusion therapy, exposing them to the associated risks of coronary dissections and perforations, arterial punctures with bleeding complications, contrast associated nephropathy, and early diagnostic closure
- Myers et al (2020) published a retrospective chart review of 467 high-risk ACS patients. They found that 40% of OMI did not present with STEMI criteria on ECG (STEMI(–) OMI). These patients suffered significant delays to cardiac catheterization despite having clinical, laboratory, and echo features of similar severity as the STEMI(+) OMI group.
The common culprits
- Publications since the 2000s describe ECG patterns without ST-segment elevation that signify acute coronary occlusion, and although there is increasing awareness of these patterns, they are yet to be included in formal diagnostic criteria and thus medical graduates and training clinicians are left with the black-and-white idea that the absence of ST elevation is a reassuring sign that this is “not a major coronary event”
- We discuss five examples of ECG patterns that every clinician should look for in the acute chest pain ECG and which, if identified, should prompt cardiology consultation for consideration of immediate PCI
OMI Example 1
Inferior wall MI
Elevation of any degree in two contiguous inferior leads with any amount of ST depression in aVL is highly suspicious for inferior OMI
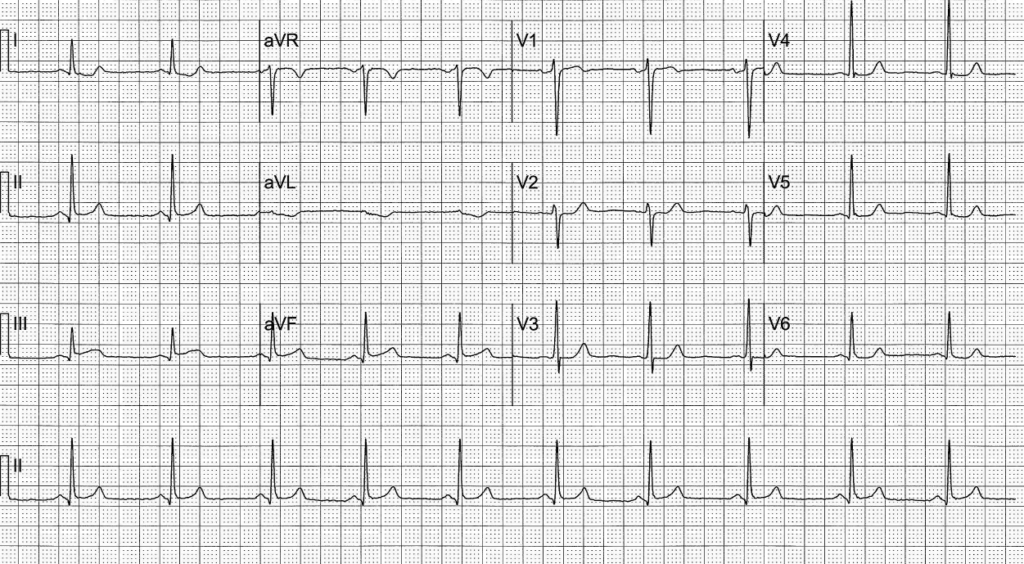
- This ECG of a 52-year-old man with chest pain demonstrates STE in inferior leads not meeting STEMI criteria, but co-existent ST depression in aVL is concerning for inferior OMI. He went on to have delayed angiography demonstrating a 95% RCA stenosis
- aVL is the only lead truly reciprocal to the inferior wall, as it is the only lead facing the superior part of the left ventricle
- The significance of aVL changes in inferior OMI can be found mentioned as early as 1993 in the European Heart Journal, where a retrospective review found that ST depression in aVL was more prevalent than STE in inferior leads in a cohort of patients with inferior OMI. In 7.5% of patients with acute inferior OMI, ST depression in aVL was the only ECG sign of infarction
- A 2016 retrospective study compared patients presenting with inferior STE that received final diagnoses of either inferior STEMI or pericarditis. Co-existing presence of ST depression in aVL was found to be highly sensitive for inferior STEMI (confidence interval 98-100%)
- A third population group in the same 2016 paper was reviewed, consisting of “subtle” STEMIs that showed occlusion on PCI but did not meet STEMI criteria. Of these, 91% demonstrated some degree of ST depression in aVL
OMI Example 2
New bifascicular block
New RBBB and LAFB is highly associated with proximal LAD occlusion and negative outcomes. Raise suspicion for OMI, and look for subtle ST changes which may be more difficult to discern.
The following ECG is from a case of a 73-year-old man who presented with 6 hours of ischaemic-sounding chest pain. His medical history included an abdominal aortic dissection two years prior, T2DM, and hypertension.
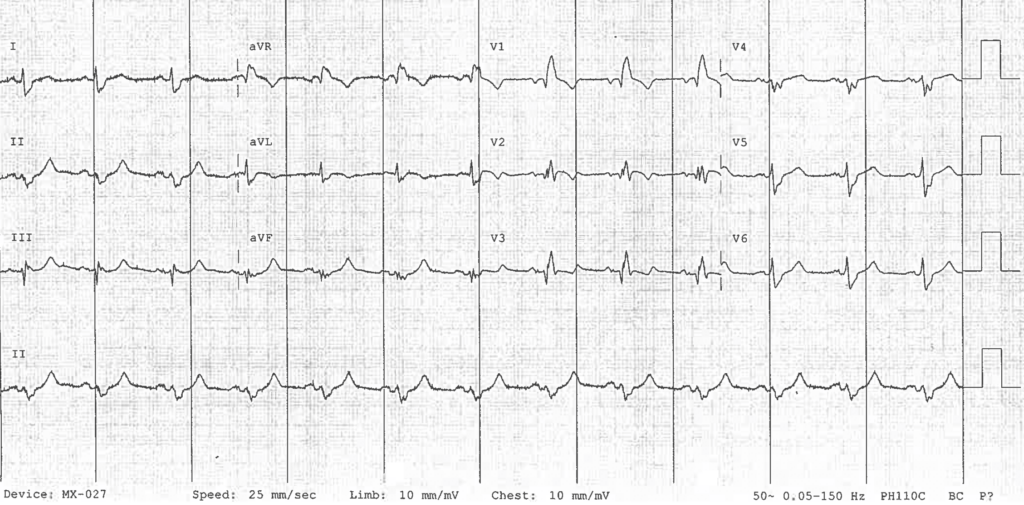
- His ECG demonstrates a RBBB + LAFB, and a segment of concordant STE in lead V2, all concerning for a proximal LAD occlusion
- Prompt cardiology referral was made with a push for immediate PCI, however there was reluctance from the consultant and a decision to await troponin levels
- Previous ECGs were found from two months prior demonstrating a normal sinus rhythm with no previous RBBB or LAFB. This further raised suspicion for an OMI
- Troponin levels returned at 2500 but a decision was made that the presentation was likely from an aortic dissection and so the patient went for CTA
- CTA showed a 100% proximal LAD occlusion and the patient was taken immediately to the cath lab
The evidence
- Autopsy studies have demonstrated that the proximal LAD septal perforators perfuse the right bundle branch and anterior fascicle of the left bundle branch in 90% of cases, while the right coronary artery perfuses the posterior fascicle of the left bundle branch in 90% of cases
- Shaikh et al advocate that new onset RBBB in ACS should be an indication for immediate reperfusion therapy as it is concerning for proximal LAD occlusion
- ESC guidelines suggest primary PCI for persisting ischaemic symptoms in the context of RBBB
- A 2011 study published in the European Heart Journal reviewed 6742 patients with AMI and found TIMI-0 flow in infarct-related territory in 52% of RBBB patients. In-hospital mortality was highest (18.8%) in patients presenting with new RBBB. The majority of cases of RBBB with acute LAD occlusion had co-existing LAFB on the admission ECG.
- Hirano et al found that 30% of LAD occlusions present with no STE, and that RBBB + LAD is typical for this catastrophic type of MI
OMI Example 3
Small “hyperacute” T-waves (HATW)
T waves out of proportion of preceding R waves, especially in the context of STE and/or reciprocal changes, should raise suspicion of OMI and impending classic STE changes
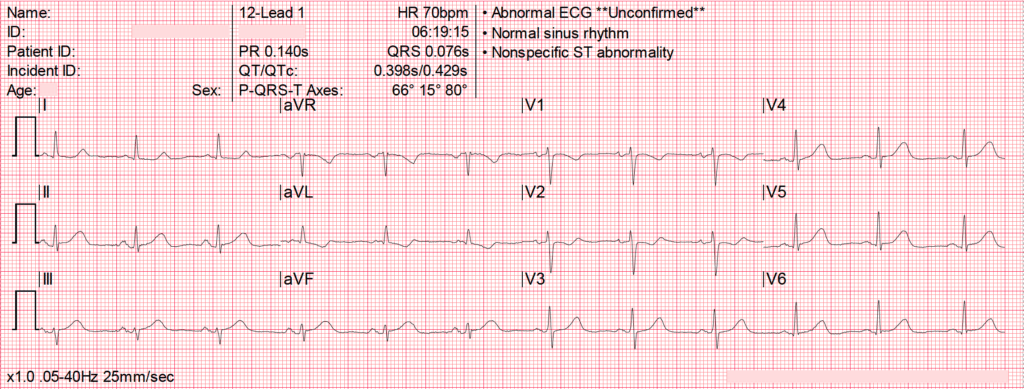
- This ECG from @tbouthillet shows HATW in inferior leads concerning for early inferior OMI, demonstrated best by their size relative to the preceding QRS Complex
- There is no formal, universal definition of what represents a HATW, however it is recognised that the ratio of T wave amplitude to the preceding complex is of more significance than overall T wave size. HATWs are wider and generally more symmetric than normal T-waves
- Serial ECGs should be performed as these changes generally precede classic STE findings or resolve if there is spontaneous reperfusion
- Experimental animal studies demonstrate that ligature of the LAD in a closed chest animal increases the voltage and width of T waves from V1-5 within 2 minutes of ligation
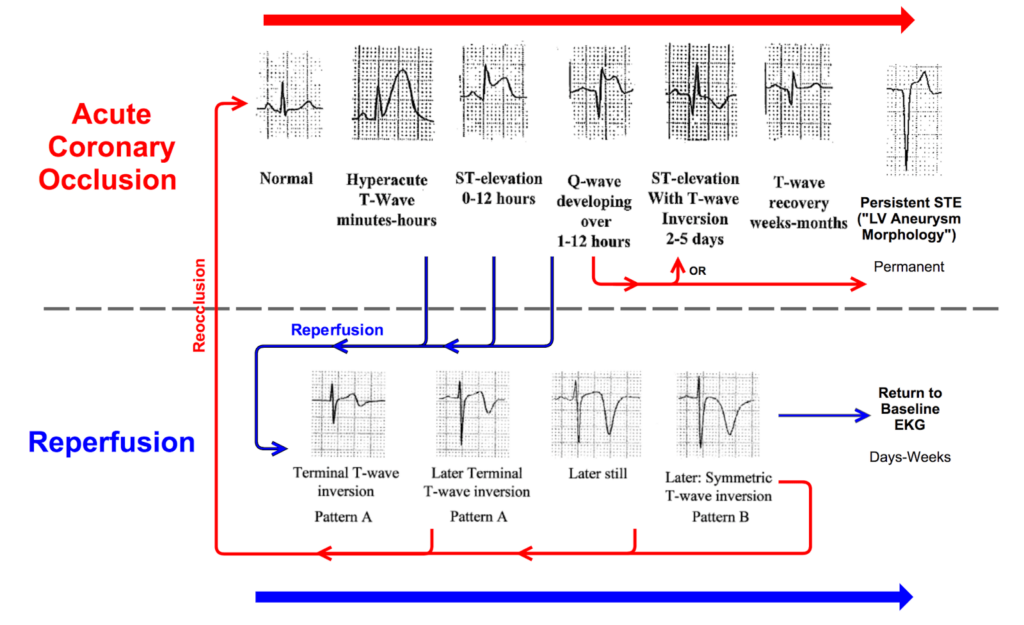
As is the case in bundle branch block, abnormal depolarisation should be followed by abnormal repolarisation. This extends to the context of a low amplitude QRS complex, which should be followed by a relatively low voltage T wave.
So how can we measure this proportion?
- Well this is where we may fall into a gap. There is little if any literature giving a clear definition of what constitutes a HATW size-wise, and we are left with a situation where the clinician must use his or her experience and pattern recognition to detect these findings concerning for OMI
- Dr Stephen W. Smith suggests that if any single T wave:QRS complex ratio in V1-4 is greater than 0.36, it represents acute MI, not subacute or old ** see comment from author (to be edited in this post)
- This is an area that warrants further retrospective analysis, especially regarding patients initially admitted with an NSTEMI diagnosis that went onto have PCI
OMI Example 4
Isolated Posterior MI
ST depression maximal in leads V1-4, without progression to V5-6, should be considered a posterior OMI until proven otherwise, even in the absence of ST elevation in leads V7-9
Posterior MI is caused by a reduction in blood flow to the dorsal, infra-atrial portion of the left ventricle. This is supplied by the posterior descending artery (PDA), a branch of the RCA in 70% of the population, the LCx in 10%, or of both in the remaining 20%. Isolated posterior MI is most commonly due to acute occlusion of the LCx.
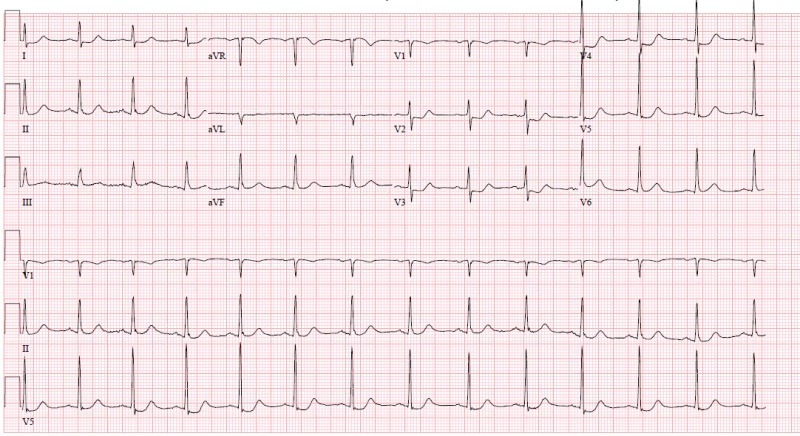
- ECG demonstrates isolated ST depression in leads V2-4, concerning for isolated posterior MI
- The patient went on to have a cath proving 100% proximal circumflex occlusion with TIMI 0 flow
- 2017 ESC guidelines recognise this pattern as suggestive of ischaemia, but only recognise it as a STEMI-equivalent if there is concomitant ST-elevation in posterior leads V7-9. It is however recognised that proximal circumflex occlusion can cause this pattern in the absence of posterior lead ST-elevation
- Electricity conducts poorly through aerated lung and thus ST elevations can be significantly reduced or absent in posterior leads, making standard ST elevation measurements inappropriate for detecting occlusion
- This isolated pattern of V1-4 involvement is contrary to subendocardial ischemia which manifests as diffuse ST depression, usually deepest in V4-V6 and lead II. This is generally a result of demand ischaemia, non-total occlusion, or sufficient collateral circulation. Be aware if there is co-existent STE in aVR (see example 5)
- Isolated posterior MI has been reported to make up at least 3% of OMIs, however this is likely an underreported figure given its common delayed or missed diagnosis
(N)OMI Example 5
Diffuse ST depression with coexistent ST elevation in aVR
Multi-lead ST depression with coexistent ST elevation in lead aVR has been described in patients with left main or proximal LAD insufficiency causing severe ischaemia.
Urgent, rather than emergent, catheterisation following medical therapy appears to be optimal management for this Non-Occlusion Myocardial Infarction (NOMI).
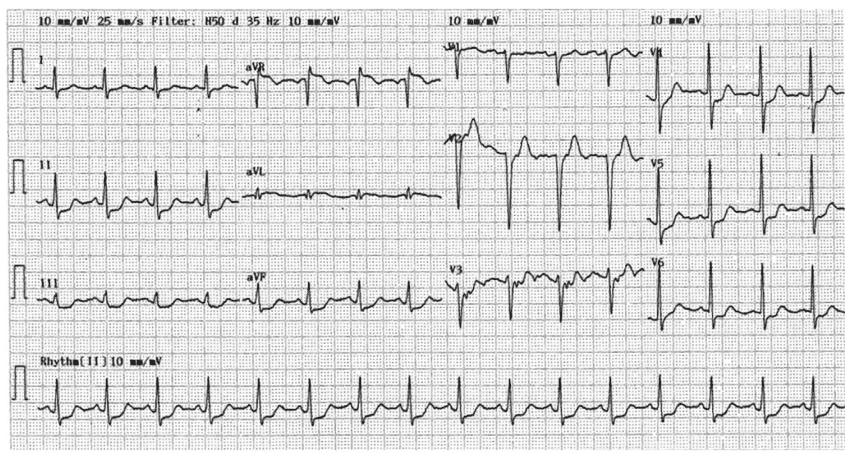
- The above ECG is that of a 51-year-old man who presented with chest pain on the background of one week of intermittent pain. aVR demonstrates STE and there is diffuse ST depression most markedly in leads II, III, and V4-6
- Coronary angiography demonstrated severe left main coronary artery disease extending into the proximal LAD and left circumflex arteries
- Previous guidelines endorsed these features as sign of acute LAD or left main coronary artery occlusion, however retrospective studies have since disputed these recommendations. More recent publications recognise the above ECG pattern as consistent with left main coronary artery subocclusion or complete occlusion with well-developed collateral circulation.
- A 2019 single-centre retrospective analysis identified patients presenting with STE-aVR with multilead ST depression. Coronary occlusion was found only in 10% of patients, and none of these lesions were involving the LAD or left main coronary artery
- Be aware of these evolving ECG features in the hypotensive, critically unwell patient, where globally reduced perfusion may manifest underlying severe coronary artery disease predisposing to ischaemia and a NOMI
aVR, the neglected lead
- Lead aVR was previously assumed to carry little diagnostic value, as it’s vector is directed away from left ventricular depolarisation. ST elevation > 1mm in lead aVR has been shown to be 80% sensitive and 93% specific for left main or triple vessel disease in patients with NSTEACS
- Proposed mechanisms of STE in aVR have been that of reciprocal changes to lateral ST depression, or more specifically, transmural ischaemia in the basal portion of the interventricular septum (LAD), or transmural ischaemia in the RVOT caused (RCA)
Where to now?
Development of an “OMI criteria” representing patients that will benefit from immediate reperfusion therapy will allow us to move away from the black-and-white idea that ST elevation is the only indicator of acute coronary occlusion
Current STEMI criteria leaves the clinician restricted in early-decision making processes if specific measurements are not met, and associated exclusion criteria in some centres such as acute pulmonary edema are manifestations of an acute, potentially reversible, disease process. The term “STEMI-equivalent” is outdated and really represents a branch of OMI criteria.
Increasing prevalence of immediate, emergent, and delayed PCI for patients admitted with myocardial infarction presents the opportunity for a large-scale retrospective analysis of ECG patterns other than ST elevation that are suggestive of occlusion.
Abnormal depolarisation should be followed by abnormal repolarisation. Infarction in a patient with low voltage QRS complexes may produce subtle ST segment changes that are best measured in relation to the preceding QRS complex. In such patient groups standard STEMI criteria would delay or potentially miss opportunities for reperfusion. Along with the HATW, a proportional measurement or criteria in relation to the preceding complex or criteria may be more appropriate for detecting occlusion. As Hirano et al describe, the different manifestations of acute LAD occlusion such as RBBB and LAFB warrant further specific analysis in large patient populations.
References
- Massie E. Chapter 6: Ventricular repolarization; ventricular gradient and spatial QRS-T angle. In: Clinical vectorcardiography and electrocardiography. 1960: 70-85
- Pinto IJ, Nanda NC, Biswas AK, Parulkar VG. Tall upright T waves in the precordial leads. Circulation. 1967; 36(5): 708-716.
- Frink RJ, James TN. Normal blood supply to the human His bundle and proximal bundle branches. Circulation. 1973; 47(1): 8-18.
- Schamroth L. Chapter 2: Electrophysiology and Electropathology. In: The electrocardiology of coronary artery disease. 1980: 19-24
- Glancy DL, Jones BP. ST-segment changes: some subtle, some obvious. Proc (Bayl Univ Med Cent). 2006; 19(4): 411-412.
- Jong GP, Ma T, Chou P, Shyu MY, Tseng WK, Chang TC. Reciprocal changes in 12-lead electrocardiography can predict left main coronary artery lesion in patients with acute myocardial infarction. Int Heart J. 2006; 47(1): 13-20.
- Hirano T at al. Clinical features of emergency electrocardiography in patients with acute myocardial infarction caused by left main trunk obstruction. Circ J. 2006; 70(5): 525-529
- Simons A, Robins LJ, Hooghoudt TE, Meursing BT, Oude Ophuis AJ. Pseudonormalisation of the T wave: old wine?: A fresh look at a 25-year-old observation. Neth Heart J. 2007;15(7-8):257-9.
- van Gorselen EO, Verheugt FW, Meursing BT, Oude Ophuis AJ. Posterior myocardial infarction: the dark side of the moon. Neth Heart J. 2007; 15(1): 16-21
- Pride YB, Tung P, Mohanavelu S, Zorkun C, Wiviott SD, Antman EM, Giugliano R, Braunwald E, Gibson CM; TIMI Study Group. Angiographic and clinical outcomes among patients with acute coronary syndromes presenting with isolated anterior ST-segment depression: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) substudy. JACC Cardiovasc Interv. 2010; 3(8): 806-811.
- Kosuge M, Ebina T, Hibi K, Morita S, Endo M, Maejima N, Iwahashi N, Okada K, Ishikawa T, Umemura S, Kimura K. An early and simple predictor of severe left main and/or three-vessel disease in patients with non-ST-segment elevation acute coronary syndrome. Am J Cardiol. 2011 Feb 15;107(4):495-500
- Fiol M, Carrillo A, Rodríguez A, Pascual M, Bethencourt A, Bayés de Luna A. Electrocardiographic changes of ST-elevation myocardial infarction in patients with complete occlusion of the left main trunk without collateral circulation: differential diagnosis and clinical considerations. J Electrocardiol. 2012;45(5):487-90
- Strauss DG, Loring Z, Selvester RH, Gerstenblith G, Tomaselli G, Weiss RG, Wagner GS, Wu KC. Right, but not left, bundle branch block is associated with large anteroseptal scar. J Am Coll Cardiol. 2013; 62(11): 959-967.
- Tamura A. Significance of lead aVR in acute coronary syndrome. World J Cardiol. 2014; 6(7): 630-637.
- Ching S, Ting SM. The Forgotten Lead: aVR in Left Main Disease. Am J Med. 2015; 128(12): e11-13
- Levis JT. ECG Diagnosis: Hyperacute T Waves. Perm J. 2015; 19(3): 79
- Bayes de Luna A, Fiol-Sala M. Where is the culprit lesion? Circulation. 2016; 134: 1507–1509.
- Bischof J. ST depression in lead aVL differentiates inferior ST-elevation myocardial infarction from pericarditis. Am J Emerg Med. 2016; 34(2): 149-154
- Ibanez B et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Kardiol Pol. 2018; 76(2): 229-313.
- Meyers PH, Weingart S, Smith SW. The OMI Manifesto. Dr Smith’s ECG blog 2018
- Miranda DF, Lobo AS, Walsh B, Sandoval Y, Smith SW. New Insights Into the Use of the 12-Lead Electrocardiogram for Diagnosing Acute Myocardial Infarction in the Emergency Department. Can J Cardiol. 2018 Feb;34(2):132-145
- Zhao YT. Total occlusion of the left main coronary artery presenting as ST-elevation myocardial infarction. J Electrocardiol. 2018; 51:479–480.
- Shaikh S et al. New Onset Right Bundle Branch Block In Acute Coronary Syndrome and High-Grade Stenosis: A Case Series. Scifed J Cardiol. 2019; 3(1): 23.
- Meyers HP, Smith SW. Prospective, real-world evidence showing the gap between ST elevation myocardial infarction (STEMI) and occlusion MI (OMI). Int J Cardiol. 2019;293:48-49.
- McLaren J. ECG Cases 4: Lateral STEMI or Occlusion MI? Emergency Medicine Cases 2019
- Meyers HP, Smith S. A New Paradigm for AMI Management. Emergency Medicine News: October 2020; 42(10): 1, 34
- Aslanger EK et al. Diagnostic accuracy of electrocardiogram for acute coronary OCClUsion resuLTing in myocardial infarction (DIFOCCULT Study). Int J Cardiol Heart Vasc. 2020; 30: 100603.
- Alblaihed L, Rezaie SR. The DIFOCCULT Trial: Time to Change from STEMI/NSTEMI to OMI/NOMI? REBEL EM
- Smith SW. The Diagnosis of OMI does not depend on the ECG. But if you recognize it, that’s great. 2020
- Meyers HP, Bracey A, Lee D, Lichtenheld A, Li WJ, Singer DD, Kane JA, Dodd KW, Meyers KE, Thode HC, Shroff GR, Singer AJ, Smith SW. Comparison of the ST-Elevation Myocardial Infarction (STEMI) vs. NSTEMI and Occlusion MI (OMI) vs. NOMI Paradigms of Acute MI. J Emerg Med. 2020 Dec 8:S0736-4679(20)31070-2
- Alblaihed L. The DIFOCCULT Trial: Time to Change from STEMI/NSTEMI to OMI/NOMI? REBEL EM
Advanced Reading
Online
- Wiesbauer F, Kühn P. ECG Mastery: Yellow Belt online course. Understand ECG basics. Medmastery
- Wiesbauer F, Kühn P. ECG Mastery: Blue Belt online course: Become an ECG expert. Medmastery
- Kühn P, Houghton A. ECG Mastery: Black Belt Workshop. Advanced ECG interpretation. Medmastery
- Rawshani A. Clinical ECG Interpretation ECG Waves
- Smith SW. Dr Smith’s ECG blog.
- Wiesbauer F. Little Black Book of ECG Secrets. Medmastery PDF
Textbooks
- Zimmerman FH. ECG Core Curriculum. 2023
- Mattu A, Berberian J, Brady WJ. Emergency ECGs: Case-Based Review and Interpretations, 2022
- Straus DG, Schocken DD. Marriott’s Practical Electrocardiography 13e, 2021
- Brady WJ, Lipinski MJ et al. Electrocardiogram in Clinical Medicine. 1e, 2020
- Mattu A, Tabas JA, Brady WJ. Electrocardiography in Emergency, Acute, and Critical Care. 2e, 2019
- Hampton J, Adlam D. The ECG Made Practical 7e, 2019
- Kühn P, Lang C, Wiesbauer F. ECG Mastery: The Simplest Way to Learn the ECG. 2015
- Grauer K. ECG Pocket Brain (Expanded) 6e, 2014
- Surawicz B, Knilans T. Chou’s Electrocardiography in Clinical Practice: Adult and Pediatric 6e, 2008
- Chan TC. ECG in Emergency Medicine and Acute Care 1e, 2004
LITFL Further Reading
- ECG Library Basics – Waves, Intervals, Segments and Clinical Interpretation
- ECG A to Z by diagnosis – ECG interpretation in clinical context
- ECG Exigency and Cardiovascular Curveball – ECG Clinical Cases
- 100 ECG Quiz – Self-assessment tool for examination practice
- ECG Reference SITES and BOOKS – the best of the rest
ECG Library
asdfasdf

ECG LIBRARY
MBBS DDU (Emergency) CCPU. Adult/Paediatric Emergency Medicine Advanced Trainee in Melbourne, Australia. Special interests in diagnostic and procedural ultrasound, medical education, and ECG interpretation. Co-creator of the LITFL ECG Library. Twitter: @rob_buttner


Great article!! A couple quick points.
My rule for T/QRS ratio with one lead of V1-V4 having ratio > 0.36 is only for differentiating the STE of “LV aneurysm” (also known as “persistent ST Elevation after old MI) from that of acute OMI.
This is an even more important rule: differentiating minimal STE in V2-V4 due to LAD OMI from that due to baseline (normal variant) STE (also frequently called “early repolarization”). I have a big post on this with lots of examples:
There is also the Smith modified Sgarbossa criteria for OMI in LBBB; see both the 1) Derivation and 2) Validation
The Smith modified Sgarbossa criteria also work for OMI in Ventricular Paced Rhythm. Presented at 2018 SAEM and almost certainly will be published in Annals of EM soon (about to re-submit revisions)
This article summarizes all the various findings of OMI, with lots of example:
One more link: This links to an abstract in Circulation. We are trying to get the manuscript published:
Im a little confused at this statement. ‘’Hirano et al found that 30% of LAD occlusions present with no STE, and that RBBB + LAD is typical for this catastrophic type of MI’’……is it meant to be RBBB+LAFB instead of RBBB + LAD?
LAD as in left axis deviation. LAFB will present as LAD. Axis deviation is how you know to check for a fascicular block in the setting of a rbbb (or ever, really, until you get good enough to just see it right away). LAD for LAFB, RAD for LPFB.
Hi guys,
Great article, thanks!
One correction: “Dr Stephen W. Smith suggests that if any single T wave:QRS complex ratio in V1-4 is greater than 0.36, it represents acute MI, not subacute or old.”
This rule only applies to this situation: a patient has ST Elevation in V2-V4 and there are Q-waves suggesting that it is “LV aneurysm morphology.”
This is also known as “persistent ST Elevation after old MI.” There are usually QS-waves. Old MI has small T-waves. Acute MI has large (tall, wide) T-waves. And so I derived and validated a rule to differentiate Acute OMI with STE from Old MI with persistent STE. If any of leads V1-V4 has a T/QRS ratio > 0.36, then it is acute. If all are less than 0.36, then it is either old or subacute. So, if chest pain has been present for > 6 hours, a low ratio could be a false negative.
I know it’s complicated, but I have used this rule myself countless times and it is very accurate.
Steve
[…] Replacing the STEMI misnomer […]