ECG Case 129
A 41-year-old man presents with one day of central, crushing chest pain, exertional in nature. He has no past medical history. HR 90 reg, BP 157/62, SpO2 100 RA.
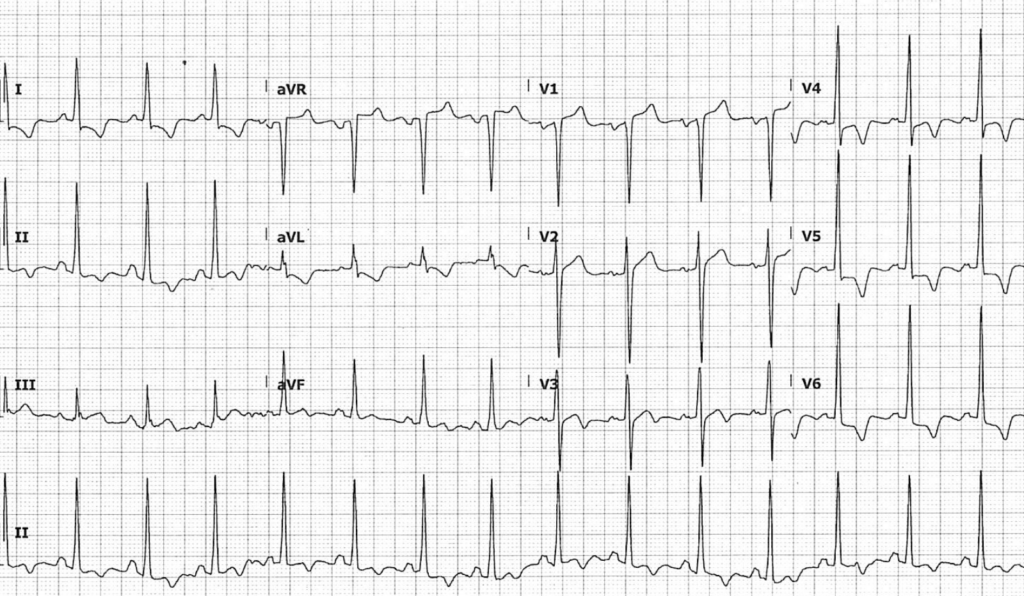
Describe and interpret this ECG
ECG ANSWER and INTERPRETATION
- Normal sinus rhythm, rate 90 BPM
- Borderline intraventricular conduction delay, with QRS duration ~100ms
- Voltage and non-voltage criteria are satisfied for left ventricular hypertrophy:
- S wave depth in V1 + tallest R wave height in V5-6 is ~45mm
- ST depression and T wave inversion in lateral leads
- Minimal ST elevation in leads V1-2 is acceptable in the context of repolarisation abnormalities due to LVH
His initial troponin returns at 54ng/L (normal < 26).
Pain resolves and a repeat ECG is taken:
What is your interpretation?
Reveal answer
- Voltage and non-voltage criteria are again met for LVH
- T-wave inversion is significantly more pronounced, with the appearance of “giant” T-wave inversion in I and V4-6 — whilst these changes can be seen in ischaemia, in this patient age group they are suspicious for apical hypertrophic cardiomyopathy (HCM)
You perform a basic bedside echo to further your assessment:
Parasternal long axis (PLAX)
Parasternal short axis (PSAX)
Apical 4 chamber (A4C)
What is your interpretation?
Reveal answer
Parasternal long axis (PLAX) view
- The apex is incompletely imaged, but there is severe left ventricular (LV) hypertrophy of both the mid-anteroseptal and apical regions, with LV cavity obliteration in systole
- Apical LV wall thickness was measured at 23mm
Parasternal short axis (PSAX) view
- PSAX view just proximal to apex, with papillary muscle partially in view
- Marked LV wall thickening and cavity obliteration is evident
Apical four chamber (A4C) view
- Image quality is suboptimal — poor endocardial definition means wall thickening is not evident
- Right ventricular size and function appears normal
OUTCOME
Coronary angiogram was unremarkable revealing smooth coronaries. Formal inpatient echo demonstrated severe LV hypertrophy, with “extreme” thickening at the apical region consistent with apical HCM. There were no features of LV outflow tract obstruction. Serial troponins remained stable and the patient was discharged on moderate dose beta-blockers with a view to titrate to symptoms as necessary.
CLINICAL PEARLS
Apical HCM: a less familial variant that requires a higher index of suspicion
Compared with classical HCM, apical HCM is a more sporadic disease with fewer patients reporting a positive family history. Gene mutations are identified in as few as 13% of cases, compared with 40% in the “classical” form of HCM that causes asymmetrical septal hypertrophy.
“Giant” negative T waves (> 10mm) are characteristic but not mandatory for diagnosis, with only half of patients presenting with this typical ECG feature.
The absence of “dagger” Q waves means that the ECG of apical HCM can easily be mistaken for uncomplicated LVH. In addition, most cases of apical HCM do not manifest LV outflow obstruction, resulting in fewer presentations of exertional pre-syncope or syncope. Typical diagnostic criteria for HCM are therefore less applicable to the apical variant, and a higher index of suspicion is required for diagnosis.
There are both “pure” (isolated apical) and “mixed” (apical and septal) forms of disease — our patient above has evidence of anteroseptal hypertrophy on echo indicating a mixed form. Note how the LV wall fails to taper off at the apex as it usually does, instead persisting in size or even thickening. Some describe the apical cavity obliteration seen in the pure form as reflecting the shape of an “ace of spades” (hover over image and drag slider to reveal)


Note that imaging of the apex with echo can be challenging. Cardiac MRI is a more sensitive diagnostic tool for detecting subtle apical abnormalities suggestive of early disease.
Inducible ischaemia in apical HCM
As seen in our patient above, severe LV apical thickening can lead to complete cavity obliteration in systole. Such obliteration can result in dynamic small vessel-obstruction in apical segments, causing recurrent ischaemic chest pain with an associated troponin rise. Prolonged, ineffective apical contraction (often into mid-diastole) further exacerbates this ischaemia.
What about our first ECG?
LV voltage criteria are identical between our two ECGs, with the only difference being the depth of T wave inversion. There are two possible explanations for this. One is that in our first ECG, a relative tachycardia results in less pronounced repolarisation abnormalities. The other is that with inducible ischaemia, there is a small degree of “pseudonormalisation” of T wave inversion. Either way, we know that diagnosing OMI in the context of LVH is very difficult and relies mainly on serial ECGs and clinical suspicion. There are no specific criteria for diagnosing OMI in the presence of LVH as there are for LBBB.
- Sporadic genetic mutations, lack of “dagger” Q waves, and an absence of LVOT obstruction means a high index of suspicion is required to diagnose apical HCM
- We must be cautious of diagnosing LVH in the absence of inciting stimuli. HCM is not necessarily a “young person” disease — the mean age of diagnosis is 41
- Inducible ischaemia is common in apical HCM, leading to recurrent exertional chest pain that requires careful symptomatic management
Further reading
Related topics
Expert Review
- Smith SW. Hypertrophic Cardiomyopathy or Normal Variant?. Dr Smith’s ECG Blog. 2019 January
References
- Arad et al. Gene mutations in apical hypertrophic cardiomyopathy. Circulation. 2005; 112:2805–2811
- Gruner et al. Sarcomere protein gene mutations in patients with apical hypertrophic cardiomyopathy. Circ Cardiovasc Genet. 2011; 4:288–295
- Jan et al. Apical hypertrophic cardiomyopathy: present status. Int J Cardiol. 2016; 222:745–759
- Flett et al. Diagnosis of apical hypertrophic cardiomyopathy: T‐wave inversion and relative but not absolute apical left ventricular hypertrophy. Int J Cardiol. 2015; 183:143–148
- Eriksson et al. Long‐term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002; 39:638–645
- Stephenson et al. Ineffective and prolonged apical contraction is associated with chest pain and ischaemia in apical hypertrophic cardiomyopathy. Int J Cardiol. 2018; 251:65–70
- Moon et al. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non‐diagnostic echocardiography. Heart. 2004; 90:645–649
Do you have an interesting ECG case? Submit it here for discussion on the blog
TOP 150 ECG Series
MBBS DDU (Emergency) CCPU. Adult/Paediatric Emergency Medicine Advanced Trainee in Melbourne, Australia. Special interests in diagnostic and procedural ultrasound, medical education, and ECG interpretation. Co-creator of the LITFL ECG Library. Twitter: @rob_buttner
Interventional cardiologist, ECG and hemodynamics fan. MD, Assoc. Prof. at Marmara University, Pendik T&R Hospital, Assoc. Editor at Archives of TSC, ESC National Prevention Coordinator





I’m under the impresion that the apical segments and the apex, though not clearly visualised, are hypocinetic…