Cryoprecipitate
Aka. Cryo
DESCRIPTION
- Fractionated plasma product consisting of Fibrinogen (Factor I), von Willebrand Factor, Factor VIII, and small amounts of Factor XIII and Fibronectin
INDICATIONS
- Congenital or acquired hypo- or afibrinogenaemia
- In a bleeding patient, with fibrinogen level less than 1.5 g/L (2.0 g/L in obstetric haemorrhage or cardiothoracic haemorrhage)
- Because the ROTEM/TEG algorithm told you to give it
ADMINISTRATION / DOSING
- Intravenous (IV)
- Adults:
- 1 unit per 5-10 kg of body weight should increase fibrinogen level by 0.5-1.0 g/L
- Typical adult dose is 10 units, approximately equivalent to 3-4 g of fibrinogen
- Children (from RCH guidelines):
- 5 – 10 mL/kg
- Administer at 10 – 20 mL/kg/h
- Administration:
- Mix thoroughly by inversion prior to use and transfuse through a blood administration set incorporating a standard 170-200 µm filter (most pump sets include a 200 µm filter)
- Administer units as fast as you need to (except in paediatrics)
- Units must be used within four hours after removal from storage
- Do not administer amongst other blood products, flush with 0.9% sodium chloride
- The half-life of fibrinogen is 3-5 days
PREPARATION
- Cryoprecipitate is prepared by thawing whole blood-derived Fresh Frozen Plasma (FFP) used for cryoprecipitate production and recovering the precipitate
- The cold-insoluble precipitate is then re-suspended in a small amount of residual plasma (15-20mL) and then re-frozen
PHARMACEUTICS
- Presentation:
- Whole Blood: Volume 30-40 mL
- Apheresis: Volume 54-55 mL
- Contents:
- Whole blood Cryo:
- Fibrinogen (Factor I) 357 (+/- 110) mg/unit
- Factor VIIIc 151 (+/- 30) IU/unit
- vWF 294 (+/- 69) IU/unit
- Apheresis Cryo:
- Fibrinogen (Factor I) 1185 (+/- 318) mg/unit
- Factor VIIIc 418 (+/- 94) IU/unit
- vWF 670 (+/- 138) IU/unit
- Whole blood Cryo:
- Storage:
- -25oC or belowThawed cryoprecipitate should be maintained at 20-24oC until transfused12-month shelf-life
- Once thawed it should be used within 6 hours if it is a closed single unit, or four hours if it is an open unit or it has been pooled
GROUP / CROSSMATCH
- Compatibility tests are not necessary, however, preferably ABO compatible with the recipients red cells
- ABO-incompatible cryoprecipitate can be used with caution – particularly in large volumes
- If large volumes of ABO-incompatible cryoprecipitate are used, the recipient may develop a positive direct antiglobulin test and, very rarely, develop haemolysis.
- Plasma components that have low titre anti-A or anti-B pose a lower risk of haemolysis.
- Matching with RhD is not necessary
ADVERSE EFFECTS
- Please see transfusion risks / reactions
- Early
- Fever
- Allergy: Mild –> Anaphylaxis
- Air embolism
- Hypothermia
- Acute haemolytic reaction
- TRALI (Transfusion-related acute lung injury)
- Volume Overload
- Citrate Toxicity
- Late
- Viral infection
- Bacterial infection
- Parasitic infection:
- Prion infection:
- GVHD (Graft versus Host Disease)
- Immune sensitisation (Rh D antigen)
- TRIM (Transfusion-related immunomodulation)
LOCATION OF ACTION
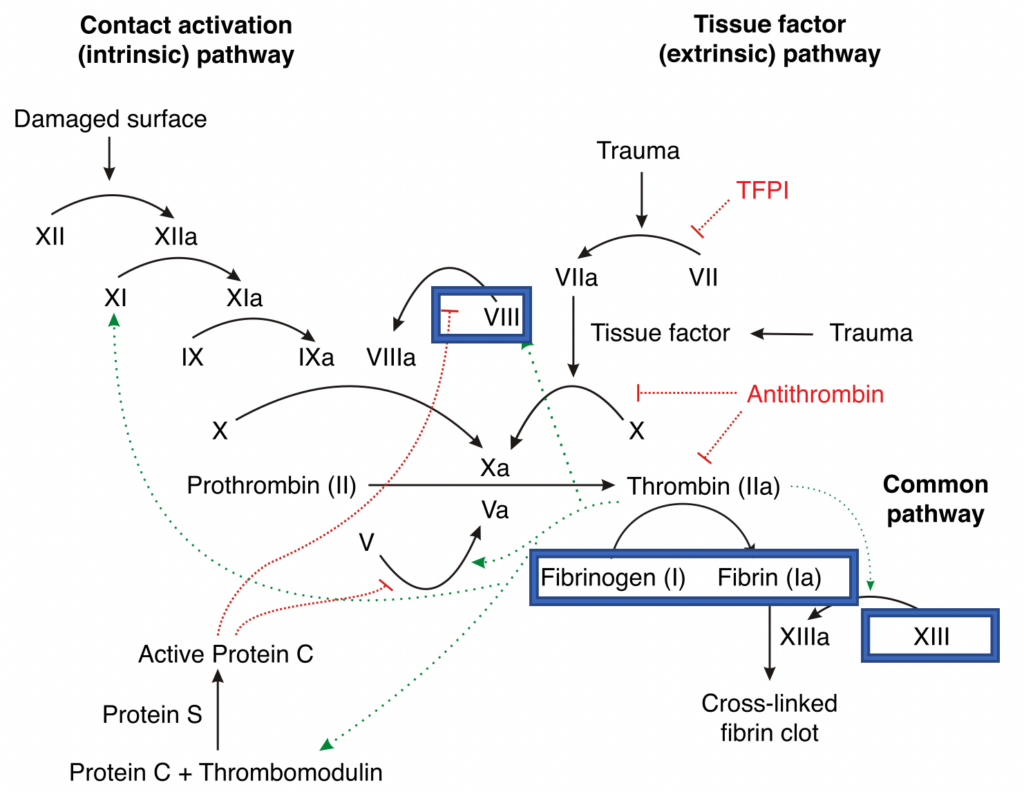
See also, Cell-Based Model of Coagulation:
MECHANISM OF ACTION
- vWF mediates platelet adhesion to damaged vascular subendothelium and thus platelet aggregation (N.B. learn about the Cell-Based Model of Coagulation above)
- Fibrinogen (factor I) in the presence of thrombin (factor IIa) and factor XIIIa (fibrin-stabilising factor) and calcium ions is converted into a stable and elastic three-dimensional fibrin (factor Ia) haemostatic clot
CONTRAINDICATIONS
- Should not be used to treat haemophilia, von Willebrand’s disease or deficiencies of Factor XIII or fibronectin unless alternative therapies are unavailable
ADVANTAGES AND DISADVANTAGES TO FIBRINOGEN CONCENTRATE
Advantages
- Cheap, roughly half the cost of fibrinogen concentrate (AUD$863.12 per 1g RiaSTAP vs AUD$163.01 per unit of cryo)
Disadvantages
- Needs to be thawed
- Some grouping / cross-match should be undertaken, especially if large volumes are to be used
- Impractical if a large quantity is needed:
- e.g. to give 1 g of fibrinogen in cryoprecipitate it would take ~3 units (= ~100 mL), or for FFP you would require ~2 units for 1 g = ~600 mL volume
- At Institution 1 (a quaternary hospital with ~600 beds in Sydney) there are 80 units of O and A each, 70 of B and 30 of AB cryoprecipitate at any one time
- e.g. to give 1 g of fibrinogen in cryoprecipitate it would take ~3 units (= ~100 mL), or for FFP you would require ~2 units for 1 g = ~600 mL volume
- Short shelf-life of 12 months (compared with 60 months)
References and Links
CCC Transfusion Series
Blood Products: Cryoprecipitate, Fresh Frozen Plasma (FFP), Platelets, Red Cells (RBCs)
>>> Factor Concentrates: Prothrombinex, Factor VIIa, Fibrinogen Concentrate
Reversal Agents:
>>> Rivaroxaban / Apixaban / Enoxaparin: Andexanet Alfa, Rivaroxaban and Bleeding
>>> Dabigatran: Idarucuzimab, Dabigatran and bleeding
>>> Heparin: Protamine
>>> Warfarin: Vitamin K, FFP, PTx, Warfarin Refersal, Warfarin Toxicity
Testing: Coagulation Studies, TEG / ROTEM (Thromboelastography), Platelet function assays
Conditions: Acute Coagulopathy of Trauma, Disseminated Intravascular Coagulation (DIC), Massive Blood Loss
General Topics: Blood Bank, Blood Conservation Strategies, Blood Product Compatibilities, Blood Transfusion Risks, Massive Transfusion Protocol (MTP), Modifications to Blood Components, Procedures and Coagulopathy, Storage Lesions, TRALI, Transfusion Literature, Transfusion Reactions
FOAMEd
References
- Australian Red Cross Lifeblood. (2020, June). Blood Component Information: An Extension of Blood Component Labels. Retrieved January 27, 2023, from https://www.lifeblood.com.au/sites/default/files/resource-library/2021-12/78.-Blood_Component_Information_1.pdf
- D, J. (2007, April 22). File:coagulation full.svg. Retrieved January 17, 2023, from https://commons.wikimedia.org/wiki/File:Coagulation_full.svg
- Holland, C. (2021, August 04). The cell-based model of coagulation – learnhaem: Haematology Made Simple. Retrieved January 27, 2023, from https://www.learnhaem.com/courses/coagulation/lessons/normal-haemostasis/topic/the-cell-based-model-of-coagulation/
- Peyvandi, F., Garagiola, I., & Baronciani, L. (2011). Role of von Willebrand factor in the haemostasis. Blood transfusion = Trasfusione del sangue, 9 Suppl 2(Suppl 2), s3–s8. https://doi.org/10.2450/2011.002S PMID: 21839029; PMCID: PMC3159913. [Free Full Text]
- National Blood Authority Australia. (2023, January 01). What blood products are supplied – national product price list. Retrieved January 18, 2023, from https://www.blood.gov.au/national-product-price-list
- The Royal Children’s Hospital Melbourne. (n.d.). Ordering Blood Products. Retrieved January 27, 2023, from https://www.rch.org.au/bloodtrans/blood_provision/Ordering_Blood_Products/
[cite]

Critical Care
Compendium
ICU Provisional Fellow BMedSci [Newcastle], BMed [Newcastle], MMed(CritCare) [Sydney] from a broadacre farm who found himself in a quaternary metropolitan ICU. Always trying to make medical education more interesting and appropriately targeted; pre-hospital and retrieval curious; passionate about equitable access to healthcare; looking forward to a future life in regional Australia. Student of LITFL.
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC

