High-dose Insulin Euglycaemic Therapy
Reviewed and revised 23 August 2024
OVERVIEW
- High-dose Insulin Euglycaemic Therapy (HIET) is primarily used in the therapy of severe calcium channel blocker toxicity
- HIET may also be used for severe beta blocker toxicity and potentially other toxicities/ presentations requiring inotropic support
INDICATION
- severe calcium channel blocker toxicity with haemodynamic compromise (especially hypotension/ shock)
- inotrope in other toxic cardiogenic shock states (e.g. second line/ adjunctive therapy for severe beta-blocker overdose)
- inotrope in non-toxic cardiogenic shock states
MECHANISM OF ACTION
HIET may allow the heart to overcome the ‘metabolic starvation’ that results from calcium channel blocker toxicity (and potentially other cardiotoxic poisonings), which compounds the direct cardiotoxic effects.
Calcium channel blocker overdoses can result in:
- hypoinsulinaemia, as insulin release is dependent on calcium influx into islet beta cells through L-type calcium channels
- calcium channel blocker-induced insulin resistance
- calcium channel blockers also impair the cardiac myocyte adaptive response of shifting from using free fatty acids, their favoured “resting state” energy substrate, to carbohydrates, due to:
- impaired uptake of glucose and free fatty acids by cardiac myocytes
- inhibition of calcium-dependent mitochondrial activity required for glucose catabolism
The effects of insulin are numerous:
- increased glucose and lactate uptake by myocardial cells
- improved myocardial function without increased oxygen demand
- increased pyruvate dehydrogenase activity, thus hastening myocardial lactate oxidation and clear the cytosol of glycolytic byproducts that can impair calcium handling and cause diastolic dysfunction.
- promotes excitation–contraction coupling and contractility because increased glucose availability results in:
- increased sarcoplasmic reticulum-associated calcium ATPase activity
- increased cytoplasmic calcium concentrations
- enhanced calcium entrance into mitochondria and sarcolemma
HIET may be best used adjunctively with other measures such as catecholamines, for two reasons:
- insulin-mediated inotropy is not catecholamine-mediated, and is not affected by β blockers, so additive effects are likely
- although insulin appears to improve myocardial contractility, it has no chronotropic effect and may cause vasodilation
DOSING AND PROTOCOL
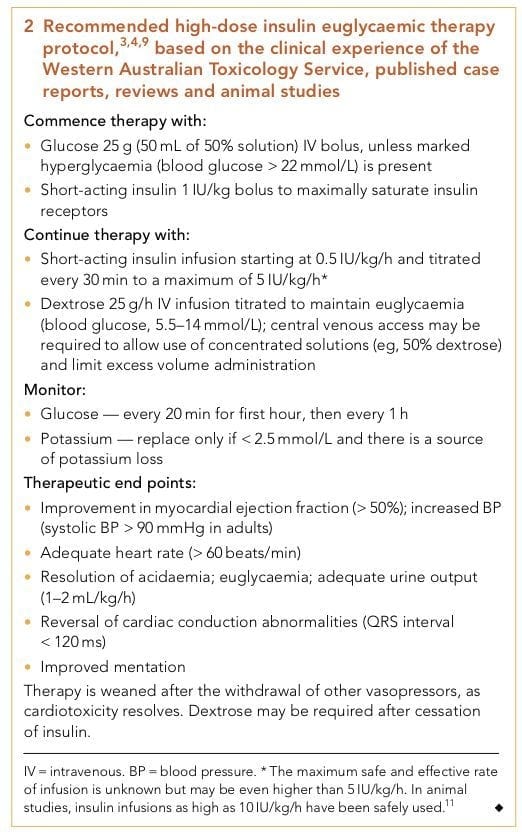
Recommended high-dose insulin euglycaemic therapy protocol based on the clinical experience of the Western Australian Toxicology Service, published case reports, reviews and animal studies (from Nickson and Little, 2009):
ADVERSE EFFECTS
Adverse events are predictable, uncommon and easily managed.
For instance, there were NO adverse effects in these extreme examples:
- the inadvertent administration of a 1000 IU insulin loading dose for verapamil toxicity
- treatment of toxic cardiogenic shock for 2 days with a 6 IU/kg/h insulin infusion
Adverse effects of HIET include:
- hypoglycaemia (<3.3 mmol/L in about 16% of cases)
- hypokalaemia
- hypomagnesaemia
- hypophosphataemia
Hypoglycemia
- Some cases of severe calcium channel blocker toxicity in patients presenting with hyperglycaemia do not require any additional glucose administration despite high-dose insulin therapy.
- hypoglycaemia may be more likely in milder cases without marked hypotension.
Hypokalaemia (potassium < 3.5 mmol/L)
- noted in only two of seven in Greene et al’s small series, with a minimum potassium level of 2.8 mmol/L.
- Excessive correction of hypokalaemia should be avoided, because it reflects the intracellular shift of potassium from the extracellular compartment due to the action of insulin, rather than a potassium-depleted state.
- Hypokalaemia in HIET may actually be beneficial:
- it may augment myocardial contractility by enhancing calcium entry during systole
- increased intracellular potassium may have a membrane-stabilising effect in excitable cells
EVIDENCE
- HIET (high-dose insulin euglycaemic therapy) was first used to treat verapamil toxicity in humans in 1993, with a favourable outcome.
- Since then, in addition to animal studies, there have been >70 cases reporting the beneficial use of HIET in humans, with an overall survival rate of 85%.
- HIET has gained widespread acceptance as a core therapy for calcium channel blocker toxicity among clinical toxicologists, even though no randomised controlled trials have been performed to test its efficacy.
References and Links
CCC Toxicology Series
General
Approach to acute poisoning, ECGs in Tox, Evidenced-based Tox, Toxicology literature summaries, Does anti-venom work?
Toxins / Overdose
Amphetamines, Barbituates, Benzylpiperazine, Beta Blockers, Calcium Channel Blocker, Carbamazepine, Carbon Monoxide, Ciguatera, Citrate, Clenbuterol, Cocaine, Corrosive ingestion, Cyanide, Digoxin, Ethanol, Ethylene Glycol, Iron, Isoniazid, Lithium, Local anaesthetic, Methanol, Monoamine oxidase inhibitor (MAOI), Mushrooms (non-hallucinogenic), Opioids, Organophosphate, Paracetamol, Paraquat, Plants, Polonium, Salicylate, Scombroid, Sodium channel blockers, Sodium valproate, Theophylline, Toxic alcohols, Tricyclic antidepressants (TCA)
Envenomation
Marine, Snakebite, Spider, Tick paralysis
Syndromes
Alcohol withdrawal, Anticholinergic syndrome, Cholinergic syndrome, Drug withdrawals in ICU, Hyperthermia associated toxidromes, Malignant hyperthermia (MH), Neuroleptic malignant syndrome (NMS), Opioid withdrawal, Propofol Infusion Syndrome (PrIS) Sedative toxidrome, Serotonin syndrome, Sympatholytic toxidrome, Sympathomimetic toxidrome
Decontamination
Activated Charcoal, Gastric lavage, GI Decontamination
Enhanced Elimination
Enhanced elimination, Hyperbaric therapy for CO
Antidotes
Antidote summary, Digibind, Glucagon, Flumazenil, HIET – High dose euglycaemic therapy, Intralipid, Methylene Blue, N-Acetylcysteine (NAC), Naloxone
Miscellaneous
Cocaine chest pain, Digoxin and stone heart theory, Hyperbaric oxygen, Hypoxaemia in tox, Liver failure in tox, Liver transplant for paracetamol, Methaemoglobinaemia, Urine drug screen
Journal articles
- Greene SL, Gawarammana I, Wood DM, Jones AL, & Dargan PI (2007). Relative safety of hyperinsulinaemia/euglycaemia therapy in the management of calcium channel blocker overdose: a prospective observational study. Intensive care medicine, 33 (11), 2019-24 PMID: 17622512
- Klein LJ, Visser FC. The effect of insulin on the heart : Part 1: Effects on metabolism and function. Neth Heart J. 2010 Apr;18(4):197-201. doi: 10.1007/BF03091761. PMID: 20428418; PMCID: PMC2856868.
Klein LJ, van Campen CM, Sieswerda GT, Kamp O, Visser FC. Effects of high-dose insulin infusion on left ventricular function in normal subjects. Neth Heart J. 2010 Apr;18(4):183-9. doi: 10.1007/BF03091759. PMID: 20428416; PMCID: PMC2856866. - Koskenkari JK, Kaukoranta PK, Kiviluoma KT, Raatikainen MJ, Ohtonen PP, Ala-Kokko TI. Metabolic and hemodynamic effects of high-dose insulin treatment in aortic valve and coronary surgery. Ann Thorac Surg. 2005 Aug;80(2):511-7. doi: 10.1016/j.athoracsur.2005.03.024. PMID: 16039195.
- Shah KR, Przybysz TM, Ushakumari D, Geib AJ. High dose insulin therapy for inotropic support during veno-arterial extracorporeal membrane oxygenation decannulation: A case report. Medicine (Baltimore). 2022 Aug 26;101(34):e30267. doi: 10.1097/MD.0000000000030267. PMID: 36042600; PMCID: PMC9410628.
- Nickson CP, & Little M (2009). Early use of high-dose insulin euglycaemic therapy for verapamil toxicity. The Medical journal of Australia, 191 (6), 350-2 PMID: 19769561 (abstract and pdf link)
FOAM and web resources
- TPR — There is no real evidence on treating calcium channel blocker overdose (2014)
- TPR — Are vasopressors effective therapy in calcium channel blocker overdose? (2013)
- TPR — Pressors or high-dose insulin for calcium channel blocker overdose? (2013)

Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC

Is HIET indicated in all CCB toxicity or only those where BP is low?
Haemodynamic compromise – e.g. hypotension and cardiogenic shock
This has been clarified in the post
Thanks!
Chris