A-a gradient
OVERVIEW
A-a gradient is calculated as PAO2 – PaO2
- PAO2 is the ‘ideal’ compartment alveolar PO2 determined from the alveolar gas equation
- The ‘short’ form of the alveolar gas equation is: PAO2 = PiO2 – PaCO2/0.8
- where PiO2 = (Patm – PH2O) x FiO2
- PiO2 is the partial pressure of oxygen inspired by the lung alveoli
- the ‘long form’ is PAO2 = (Patm – PH2O) x FiO2 – PaCO2/RQ+ f
- Patm is atmospheric pressure
- PH20 is the water vapour pressure (47mmHg if 100% humidification at body temperature, 37C)
- FiO2 is the fraction of inspired oxygen
- PaCO2 is the arterial partial pressure of CO2
- RQ is the respiratory quotient (usually 0.8 but can , and vary with diet and metabolic state)
- F is a commonly ignored correction factor of 2-3 mmHg accounting for changes in the partial pressure of nitrogen.
- where PiO2 = (Patm – PH2O) x FiO2
- A normal A–a gradient for a young adult non-smoker breathing air, is between 5–10 mmHg.
- However, the A–a gradient increases with age (see limitations)
CLASSIFICATION OF HYPOXIA BASED ON A-a GRADIENT
Normal A-a gradient
- Alveolar hypoventilation (elevated PACO2)
- Low PiO2 (FiO2 < 0.21 or barometric pressure < 760 mmHg)
Raised A-a gradient
- Diffusion defect (rare)
- V/Q mismatch
- Right-to-Left shunt (intrapulmonary or cardiac)
- Increased O2 extraction (CaO2-CvO2)
LIMITATIONS
- Gradient varies with age and FiO2:
- FiO2 0.21 – 7 mmHg in young, 14 mmHg in elderly
- FiO2 1.0 – 31 mmHg in young, 56 mmHg in elderly
- For every decade a person has lived, their A–a gradient is expected to increase by 1 mmHg – a conservative estimate of normal A–a gradient is < [age in years/4] + 4.
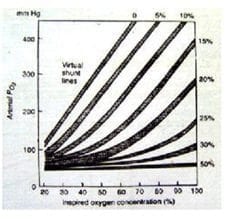
- an exaggerated FiO2 dependence in intrapulmonary shunt (PAO2 vs PAO2/PaO2 difference diagram with regard to increasing percentage of shunt) and even more so in V/Q mismatch.
References and Links
CCC Ventilation Series
Modes: Adaptive Support Ventilation (ASV), Airway Pressure Release Ventilation (APRV), High Frequency Oscillation Ventilation (HFOV), High Frequency Ventilation (HFV), Modes of ventilation, Non-Invasive Ventilation (NIV), Spontaneous breathing and mechanical ventilation
Conditions: Acute Respiratory Distress Syndrome (ARDS), ARDS Definitions, ARDS Literature Summaries, Asthma, Bronchopleural Fistula, Burns, Oxygenation and Ventilation, COPD, Haemoptysis, Improving Oxygenation in ARDS, NIV and Asthma, NIV and the Critically Ill, Ventilator Induced Lung Injury (VILI), Volutrauma
Strategies: ARDSnet Ventilation, Open lung approach, Oxygen Saturation Targets, Protective Lung Ventilation, Recruitment manoeuvres in ARDS, Sedation pauses, Selective Lung Ventilation
Adjuncts: Adjunctive Respiratory Therapies, ECMO Overview, Heliox, Neuromuscular blockade in ARDS, Prone positioning and Mechanical Ventilation
Situations: Cuff leak, Difficulty weaning, High Airway Pressures, Post-Intubation Care, Post-intubation hypoxia
Troubleshooting: Autotriggering of the ventilator, High airway and alveolar pressures / pressure alarm, Ventilator Dyssynchrony
Investigation / Indices: A-a gradient, Capnography and waveforms, Electrical Impedance Tomography, Indices that predict difficult weaning, PaO2/FiO2 Ratio (PF), Transpulmonary pressure (TPP)
Extubation: Cuff Leak Test, Extubation Assessment in ED, Extubation Assessment in ICU, NIV for weaning, Post-Extubation Stridor, Spontaneous breathing trial, Unplanned extubation, Weaning from mechanical ventilation
Core Knowledge: Basics of Mechanical Ventilation, Driving Pressure, Dynamic pressure-volume loops, flow versus time graph, flow volume loops, Indications and complications, Intrinsic PEEP (autoPEEP), Oxygen Haemoglobin Dissociation Curve, Positive End Expiratory Pressure (PEEP), Pulmonary Mechanics, Pressure Vs Time Graph, Pressure vs Volume Loop, Setting up a ventilator, Ventilator waveform analysis, Volume vs time graph
Equipment: Capnography and CO2 Detector, Heat and Moisture Exchanger (HME), Ideal helicopter ventilator, Wet Circuit
MISC: Sedation in ICU, Ventilation literature summaries
Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at the Alfred ICU in Melbourne. He is also a Clinical Adjunct Associate Professor at Monash University. He is a co-founder of the Australia and New Zealand Clinician Educator Network (ANZCEN) and is the Lead for the ANZCEN Clinician Educator Incubator programme. He is on the Board of Directors for the Intensive Care Foundation and is a First Part Examiner for the College of Intensive Care Medicine. He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives.
After finishing his medical degree at the University of Auckland, he continued post-graduate training in New Zealand as well as Australia’s Northern Territory, Perth and Melbourne. He has completed fellowship training in both intensive care medicine and emergency medicine, as well as post-graduate training in biochemistry, clinical toxicology, clinical epidemiology, and health professional education.
He is actively involved in in using translational simulation to improve patient care and the design of processes and systems at Alfred Health. He coordinates the Alfred ICU’s education and simulation programmes and runs the unit’s education website, INTENSIVE. He created the ‘Critically Ill Airway’ course and teaches on numerous courses around the world. He is one of the founders of the FOAM movement (Free Open-Access Medical education) and is co-creator of litfl.com, the RAGE podcast, the Resuscitology course, and the SMACC conference.
His one great achievement is being the father of three amazing children.
On Twitter, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC
4 Comments
Comments are closed.

I take a low Pmv02, as in heart failure, would also cause hypoxemia without an elevated (A-a) gradient?
Hi David
I think that in normal lungs at rest, that low PVO2, is unlikely to cause low PaO2
This is because PO2 100mHg is typically achieve after blood transits across a third of the lung capillaries (in 0.25 msec) from a starting PvO2 of about 40mmHg – so there is plenty of “buffer” to oxygenate further at a lower starting PvO2.
This may not be the case in extreme exercise (e.g. lung capillary transit time may be reduced to 0.25 msec) or if co-existent causes of an increased A-a gradient exist, limiting oxygenation of blood flowing theough the lungs.
Cheers
Chris
Feel free to correct me if I’m wrong but to answer David’s question, assuming that he meant heart failure as reduction in cardiac output without concomitant pulmonary effects like pulmonary oedema:
Reduction in CO does cause reduction in mixed venous O2 content (PmvO2) which would decrease PaO2 however it also tends to cause reduction in shunt fraction, so essentially you get a reduced shunt fraction of more desaturated mixed venous blood (competing effects) so PaO2 hardly changes.
IF you factor into the equation heart failure causing a degree of pulmonary oedema you’ll get an increase in shunt fraction as well (due to expansion of West Zone 4) which will reduce PaO2
Thanks for the comment John,
Chris