Open Lung Approach To Ventilation
OVERVIEW
The open lung approach (OLA) to ventilation involves increasing the level of Positive End Expiratory Pressure (PEEP) in combination with protective lung ventilation
- protective lung ventilation with low tidal volumes (4-8 mL/kg PBW) and limited plateau pressures (Pplat <30 cmH20) is now widely considered the standard of care in acute respiratory distress syndrome (ARDS)
- The “open lung concept” was a proposal to use recruitment manoeuvres followed by higher PEEP to reduce atelectrauma and shear-stress (Lachman, 1992)
- A universally accepted protocol for OLA ventilation does not exist
- The use of PEEP — at what level and how it should be optimised — remains controversial
- Recruitment manoeuvres in ARDS and novel ventilation modes (e.g. Airway Pressure Release Ventilation (APRV)) may also be used as part of OLA ventilation
RATIONALE
Potential benefits of PEEP and OLA ventilation in ARDS
- maximises recruitment of alveoli (by preventing de-recruitment)
- minimises cyclic atelectasis and atelectrauma
- decreases biotrauma from alveolar collapse (e.g. release of inflammatory mediators)
- minimises denitrogenation atelectasis and oxygen toxicity (by allowing lower FiO2)
See the Positive End Expiratory Pressure (PEEP) page for a more extensive overview of the potential advantages and disadvantages of PEEP.
DETERMINING OPTIMAL PEEP
Optimal PEEP level ultimately represents a balance between regional areas of overstretching and regional derecruitment to prevent VILI, while achieving optimal oxygenation and minimsing harmful effects such as haemodynamic compromise (Schmidt, 2012; Miller et al, 2012).
- no agreed upon ideal method of determination
- optimal PEEP may:
- change over time in an individual patient (e.g. disease progression, positioning)
- be independent of oxygenation (e.g. due to hyperinflation injury) as increased oxygenation does not correlate with improved alveolar stability (Nieman et al, 2019; Schmidt, 2012)
- be independent of respiratory mechanics
- Certain patients groups may have different PEEP requirements
- e.g. in obesity, patients typically need higher PEEP than non-obese patients to maintain alveolar recruitment
- e.g. in asthma, can use either ZEEP (PEEP = 0) or 2/3 of measured auto-PEEP (no high-level evidence for either, approach however PEEP is preferred in spontaneous ventilation modes to reduce work of breathing)
There are a number of methods suggested to determine the optimum PEEP setting, all of which have pros and cons:
- adjust according to a sliding scale of FiO2 requirements (e.g. as per ARDSNet Ventilation Strategy for protective lung ventilation)
- a simple, pragmatic approach that focus on optimising oxygenation and Plat
- PEEP settings at low FiO2 typically are lower than those suggested by PEEP optimisation strategies based on lung recruitment (e.g. Open Lung Approach To Ventilation).
- perform recruitment manoeuvre (RM) (see Recruitment manoeuvres in ARDS) then adjust to either optimal SpO2, static compliance, or other parameter listed below.
- Staircase recruitment manoeuvres are generally cautioned against due to increased mortality seen in the intervention arm of the ART trial (ART trial investigators, 2017)
- set PEEP according to pressure-volume loop analysis. Various approaches have been proposed including setting PEEP based on:
- inflation limb lower inflection point (Pflex),
- deflation limb upper Pflex,
- maximal compliance,
- true inflection point of the deflation limb,
- the degree of hysteresis, or
- stress index (a parameter derived from the PV loop, a straight line (0.95 > SI <1.05) is indicative of less injurious ventilation)
- adjust PEEP to maximise static compliance (Cstat) (increased risk of alveolar stretch above this)
- Cstat = TV / (Pplat – PEEP)
- tends to underestimate alveolar recruitment
- adjust PEEP to optimise driving pressure (implies optimal compliance, but also affected by tidal volume/ plateau pressure)
- adjust PEEP to minimise PaCO2-ETCO2 gradient (surrogate for dead space)
- guided by pulmonary computed tomography (CT)
- often impractical
- may be used to estimate lung strain (ratio of end-inspiratory volume and functional residual capacity)
- CT-derived PEEP does not correlate well with other indicators of recruitability (Cressoni et al, 2014)
- lowest intra-pulmonary shunt (highest SvO2)
- esophageal balloon directed estimation of pleural pressures to calculate transpulmonary pressures (TPP) and guide PEEP titration
- electrical impedance tomography (EIT)
- model-based methods
See Open Lung Approach To Ventilation for further discussion of PEEP in mechanical ventilation.
EVIDENCE
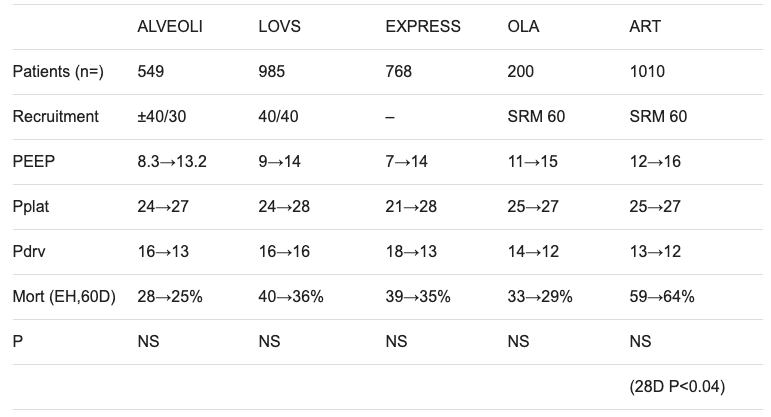
Three major studies have assessed high versus low PEEP in combination with protective lung ventilation at low tidal volumes for ARDS
- ALVEOLI trial (2004) — no difference between high and low PEEP
- LOVS trial (2008) — improved oxygenation and less need for rescue interventions (e.g. ECMO), no difference in mortality
- EXPRESS trial (2008) — more ventilator-free days and more organ failure-free days, no difference in mortality
- Briel et al (2010) systematic review and meta-analysis — used independent patient data from the above 3 trials ( 2299 patients) and showed that higher levels of PEEP were associated with improved survival among moderate-and-severe ARDS but not ALI patients (i.e. if PF ratios <200 there was 34.1% versus 39.1% mortality with adjusted RR, 0.90; 95% CI, 0.81-1.00; P = 0.049)
- Laffey et al (2016) observational study – this study involved 2377 patients from 459 ICUs from 50 countries and found that for moderate ARDS, patients with a lower PEEP (<12 cmH2O) had a risk of hospital mortality 26 % greater than those observed in patients with higher PEEP [RR 1.26 (95 % CI 1.00–1.58)]. This was not seen in mild or severe ARDS.
- OLA trial (2016) — open lung approach using higher PEEP improved oxygenation and driving pressure, with no effect on mortality, ventilator-free days, or barotrauma.
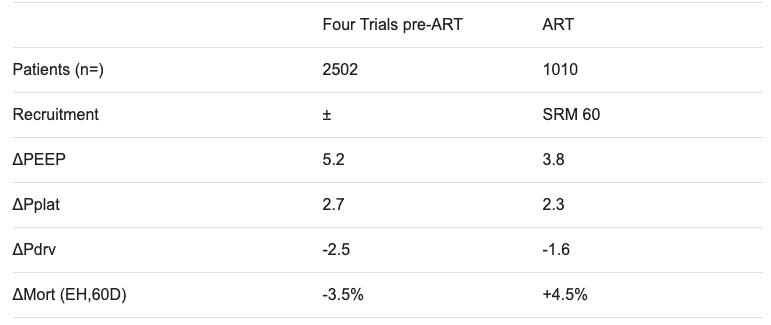
- ART trial (2017) — OLA ventilation using staircase recruitment manoeuvres (SRM) in moderate-to-severe ARDS patients increased mortality compared with protective lung ventilation (see below)
- PHARLAP trial (2019) — OLA ventilation using staircase recruitment manoeuvres (SRM) in moderate-to-severe ARDS patients did not improve VFDs or mortality compared with protective lung ventilation. The intervention was associated with increased cardiovascular events but reduced use of rescue therapies for hypoxemia (see below)
Key trial data summary tables prior to PHARLAP (courtesy of Professor David Tuxen)
ART trial, 2017
- international multicenter Randomised Controlled Trial (RCT)
- 1013 patients randomised from 120 intensive care units from 9 countries (mostly South America)
- patients included received invasive mechanical ventilation with moderate to severe ARDS (PF ratio <200) of less than 72 hours of duration
- intervention:
- high-PEEP and staircase recruitment manoeuvre (SRM) optimising static lung compliance using a protocolised approach (an open lung approach (OLA) to mechanical ventilation)
- control:
- low-PEEP and low- tidal volume Acute Respiratory Distress Syndrome Network (ARDSNet) strategy
- primary outcome:
- the intervention (OLA approach) had increased 28-day all-cause mortality (55.3% vs 49.3%)
- Commentary and criticisms:
- overall, a well-designed trial
- the control arm of the ART trial had higher PEEP (mean 2-3 cmH2O higher) than most studies using the ARDSNet ventilation strategy
- concerns include:
- generalisability to Australasian ICU setting, where the PHARLAP trial has been performed but is yet to be published
- a relatively high mortality rate in both the intervention and control arms of the trial
- the morbidity and mortality that was temporally associated with the SRM was not apparent in other trials
- is optimising static compliance the best way to set PEEP?
- would identification of “PEEP responsive” patient subgroups prior to the intervention allow only those who might benefit receive the intervention and prevent harm to “non-responders”?
- are the findings generalizable to other OLA ventilation strategies? (e.g. high PEEP vs high PEEP + SRM vs high PEEP + other recruitment manoeuvres vs high PEEP + recruitment manoeuvres targeting optimal PEEP based on parameters other than best static compliance)
PHARLAP trial, 2019
- International multicenter Randomised Controlled Trial (RCT)
- 1013 patients randomised from 35 intensive care units (ICUs) in 5 countries (Australia, Ireland, Kingdom of Saudi Arabia, New Zealand, and the United Kingdom)
- patients included received invasive mechanical ventilation ≤ 72 hours for a diagnosis of moderate – severe ARDS as defined by the Berlin conference criteria
- intervention:
- pressure controlled ventilation (Pinsp 15 +/- 3 cmH2O to achieve a goal VT 4 – 6 mL/Kg PBW) and daily combined open lung procedure (COLP) consisting of:
- staircase recruitment manoeurve (SRM) (described in Lung recruitment manoeuvres in ARDS)
- PEEP titration down to the “derecruitment PEEP” identified by a 2% decrease in SpO2 (or a minimum PEEP of 15 cmH20)
- Brief recruitment manoeurvre (2 minute RM with PEEP set at maximum tolerated PEEP achieved during SRM) then PEEP reduced to optimum setting (2.5 cmH20 above the “derecruitment PEEP”)
- pressure controlled ventilation (Pinsp 15 +/- 3 cmH2O to achieve a goal VT 4 – 6 mL/Kg PBW) and daily combined open lung procedure (COLP) consisting of:
- control:
- volume controlled ventilation using the low-PEEP and low- tidal volume Acute Respiratory Distress Syndrome Network (ARDSNet) strategy
- primary outcome:
- no difference between intervention and control arms in Ventilator Free Days at day 28 (16 (IQR 0 – 21) vs 14.5 (0 – 21.5), p = 0.95)
- secondary outcomes:
- no statistically significant difference in mortality (up to 6 months), duration of ventilation, ICU or hospital length of stay
- the intervention had higher rates of new cardiac arrhythmia (30% vs 12.5%, p = 0.03) but not severe hypotension: (34.5% vs 21.4%, p = 0.12), or barotrauma (5.2% vs 10.7%, p = 0.32)
- the intervention had lower rates of rescue therapies for hypoxaemia: ECMO (1.7% vs 12.5%, p = 0.03), prone positioning (6.9% vs 12.5% (P = 0.02), and inhaled nitric oxide (10.3% vs 28.6%, p = 0.03).
- Commentary and criticisms:
- well designed trial with low risk of systematic bias, except for lack of blinding (intention-to-treat analysis, good baseline balance, high protocol adherence, no loss to follow up)
- trial was stopped early due to loss of equipoise after the publication of the ART trial and was underpowered
- high consultant involvement, who performed the COLPs (90% of the time)
- low use of prone ventilation in the intervention arm
- generalisable to the ANZ setting, with mortality ~25% in both arms
- similar issues to ART: what is the best PEEP optimisation strategy, are there PEEP “responders” and “non-responders”?
- it is possible that if COLPs are performed by senior staff, in patient’s with no contra-indications, they may be safe and reduce the need for other rescue therapies
The effect of low VT ventilation with higher levels of PEEP in patients without ARDS is uncertain
CONCLUSION
The ARDSNet protective lung ventilation protocol remains the standard of care for the mechanical ventilation of ARDS patients. However ATS/ESICM/SCCM guidelines recommend the use of higher, rather than lower PEEP, in the management of ARDS (Fan et al, 2017).
Use of higher PEEP than traditionally prescribed by the ARDSNet protective lung ventilation protocol is reasonable if tailored to individual patient requirements, but OLA ventilation with recruitment manoeuvres should not be routinely used in the amnagement of ARDS.
- Higher PEEP (>15 cmH20) is a reasonable approach in patients with the highest lung recruitability and in the most hypoxemic patients
- A meta-analysis (pre-ART trial) suggests a possible mortality benefit of protective lung ventilation with high PEEP in patients with moderate-to-severe ARDS (PF ratio <200)
- Even the control arm of the ART trial had higher PEEP (mean 2-3 cmH2O higher) than most studies using the ARDSNet ventilation strategy
- The method of determination of optimal PEEP is controversial
- Specific subgroups of ARDS patients may benefit from OLA ventilation with recruitment manoeuvres, but identification of these subgroups is uncertain, the evidence is weak, and there is risk of harm to patients (as found in the ART trial).
- if recruitment manoeuvres are performed, staircase recruitment manoeuvres (SRMs) may be best avoided due to the potential risk of harm and increased mortality
References and Links
CCC Ventilation Series
Modes: Adaptive Support Ventilation (ASV), Airway Pressure Release Ventilation (APRV), High Frequency Oscillation Ventilation (HFOV), High Frequency Ventilation (HFV), Modes of ventilation, Non-Invasive Ventilation (NIV), Spontaneous breathing and mechanical ventilation
Conditions: Acute Respiratory Distress Syndrome (ARDS), ARDS Definitions, ARDS Literature Summaries, Asthma, Bronchopleural Fistula, Burns, Oxygenation and Ventilation, COPD, Haemoptysis, Improving Oxygenation in ARDS, NIV and Asthma, NIV and the Critically Ill, Ventilator Induced Lung Injury (VILI), Volutrauma
Strategies: ARDSnet Ventilation, Open lung approach, Oxygen Saturation Targets, Protective Lung Ventilation, Recruitment manoeuvres in ARDS, Sedation pauses, Selective Lung Ventilation
Adjuncts: Adjunctive Respiratory Therapies, ECMO Overview, Heliox, Neuromuscular blockade in ARDS, Prone positioning and Mechanical Ventilation
Situations: Cuff leak, Difficulty weaning, High Airway Pressures, Post-Intubation Care, Post-intubation hypoxia
Troubleshooting: Autotriggering of the ventilator, High airway and alveolar pressures / pressure alarm, Ventilator Dyssynchrony
Investigation / Indices: A-a gradient, Capnography and waveforms, Electrical Impedance Tomography, Indices that predict difficult weaning, PaO2/FiO2 Ratio (PF), Transpulmonary pressure (TPP)
Extubation: Cuff Leak Test, Extubation Assessment in ED, Extubation Assessment in ICU, NIV for weaning, Post-Extubation Stridor, Spontaneous breathing trial, Unplanned extubation, Weaning from mechanical ventilation
Core Knowledge: Basics of Mechanical Ventilation, Driving Pressure, Dynamic pressure-volume loops, flow versus time graph, flow volume loops, Indications and complications, Intrinsic PEEP (autoPEEP), Oxygen Haemoglobin Dissociation Curve, Positive End Expiratory Pressure (PEEP), Pulmonary Mechanics, Pressure Vs Time Graph, Pressure vs Volume Loop, Setting up a ventilator, Ventilator waveform analysis, Volume vs time graph
Equipment: Capnography and CO2 Detector, Heat and Moisture Exchanger (HME), Ideal helicopter ventilator, Wet Circuit
MISC: Sedation in ICU, Ventilation literature summaries
Journal articles
- ART investigators writing group. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA. 2017; 318(14):1335-1345. [pubmed] [article] (ART)
- Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010 Mar 3;303(9):865-73. [pubmed]
- Brower RG, Lanken PN, MacIntyre N, et al; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004 Jul 22;351(4):327-36. [pubmed] (ALVEOLI)
- Guerin C. The preventive role of higher PEEP in treating severely hypoxemic ARDS. Minerva Anestesiol. 2011 Aug;77(8):835-45. Review. [pubmed] [article]
- Kacmarek RM, Villar J, Sulemanji D, et al. Open Lung Approach for the Acute Respiratory Distress Syndrome: A Pilot, Randomized Controlled Trial. Critical care medicine. 2016; 44(1):32-42. [pubmed] (OLA)
- Lachmann B. Open up the lung and keep the lung open. Intensive Care Med. 1992;18:319–21. [pubmed]
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008 Feb 13;299(6):637-45. .[pubmed] (LOVS)
- Mercat A, Richard JC, Vielle B, et al; Expiratory Pressure (Express) Study Group. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008 Feb 13;299(6):646-55. [pubmed] (EXPRESS)
- Rouby JJ, Ferrari F, Bouhemad B, Lu Q. Positive end-expiratory pressure in acute respiratory distress syndrome: should the ‘open lung strategy’ be replaced by a ‘protective lung strategy’? Crit Care. 2007;11(6):180. Review. [pubmed] [article]
- Rubenfeld GD. How much PEEP in acute lung injury. JAMA. 2010 Mar 3;303(9):883-4. [pubmed]
- Santa Cruz R, Rojas JI, Nervi R, Heredia R, Ciapponi A. High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013 Jun 6;6:CD009098. [pubmed]
- Suter PM, Fairley B, Isenberg MD. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med. 1975 Feb 6;292(6):284-9.[pubmed]
- Villar J, Kacmarek RM, Pérez-Méndez L, et al. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006 May;34(5):1311-8. [pubmed]
FOAM and web resources
Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC

Hi id like to know what is ASB for in a BIPAP mode of ventilation? Thank you
ASB stands for assisted spontaneous breathing (not to be confused with ASV – adaptive support ventilation)
It is a mode on some ventilators, equivalent to PSV (pressure support ventilation)
A breath is spontaneous when the patient determines both the size and timing of the breath.
A breath is assisted when the ventilator does some of the work for the patient (inspiratory pressure from the ventilator increases above the end-expiratory pressure)
Thus spontaneous breathing in CPAP is unassisted, as there is no additional ventilator support on inspiration.
However, spontaneous breathing in BiPAP or PSV is assisted, because the ventilator provides additional positive pressure during inspiration, and is synchronised with the patient’s respiratory effort.
Cheers
Chris