Extracorporeal Membrane Oxygenation
OVERVIEW
- ECMO = extracorporeal membrane oxygenation
- extracorporeal life support (ECLS) may be a better term
- The extracorporeal circuit allows for the oxygenation and removal of carbon dioxide from blood
- used as a supportive strategy in patients who have a high risk of death despite conventional therapy
HISTORY
Neonates
- 1974 – used in the setting of meconium aspiration with severe hypoxaemia
- first RCT (Bartlett) – benefit shown but methodology was highly criticized
- 1993-1995, UK: ECMO vs conventional care -> trial stopped early due to benefit (NNT 3)
Adults
- 1972 – first survivor (NEJM) – young patient with post-traumatic respiratory failure
- 1979 – VA ECMO (ECMO with ventilation) vs ventilation alone -> high mortality in ECMO group
- 1986 — Gattinoni’s case series, VV, used for CO2 removal, increased survival but large blood loss/day
TYPES
- VV = veno-venous
- VA = veno-arterial: peripheral or central
- Veno-pulmonary artery ECMO (provides short-term right ventricular and respiratory support following LVAD insertion)
- high (2 venous cannulae) vs low flow (1 venous cannula)
INDICATIONS
acute, severe REVERSIBLE respiratory or cardiac failure with a high risk of death that is refractory to conventional management
- poor gas exchange
- compliance < 0.5mL/cmH2O/kg
- P:F ratio < 100
- shunt fraction > 30%
GENERAL CONTRAINDICATIONS
Absolute
- progressive non-recoverable cardiac disease (not transplant candidate)
- progressive and non-recoverable respiratory disease (irrespective of transplant status)
- chronic severe pulmonary hypertension
- advanced malignancy
- GVHD
- >120kg
- unwitnessed cardiac arrest
Relative
- age > 75
- multi-trauma with multiple bleeding sites
- CPR > 60 minutes
- multiple organ failure
- CNS injury
VV ECMO
- most common mode
- venous drainage from large central veins -> oxygenator -> venous system near RA
- support for severe respiratory failure (no cardiac dysfunction)
- pathology:
-> pneumonia
-> ARDS
-> acute GVHD
-> pulmonary contusion
-> smoke inhalation
-> status asthmaticus
-> airway obstruction
-> aspiration
-> bridge to lung transplant
-> drowning
- specific contraindications:
— unsupportable cardiac failure
— severe pulmonary hypertension
— cardiac arrest
— immunosuppression (severe)

- oxygenated blood returned to right side of heart so oxygen loss can take place through the lungs
Advantages
- normal lung blood flow
- oxygenated lung blood
- pulsatile blood pressure
- oxygenated blood delivered to root of aorta
- must be used when native cardiac output is high
Disadvantages
- no cardiac support
- local recirculation though oxygenator at high flows
- reverse gas exchange in lung if FiO2 low
- limited power to create high systemic arterial oxygen tension
Ventilation
- no need to ventilate at normal level
- must maintain alveolar volume and oxygenation
- RR 8-10, PEEP 15, TV 3-4mL/kg, PIP < 30, FiO2 0.4
- mandatory bronchoscopy and normal ventilation before decannulation
DETERMINATES OF FLOW WITH VENOUS LINES
- cannula size
- patency of vein
- suck down and kicking of line (vein collapsing around cannula)
-> fluid load
-> check intra-abdominal pressure
-> add a second drainage line
-> return cannula advanced into right ventricle (rare)
VA ECMO
- venous drainage from large central veins -> oxygenator -> arterial system in aorta
- support for cardiac failure (+/- respiratory failure)
- pathology:
-> graft failure post heart or heart lung transplant
-> non-ischaemic cardiogenic shock
-> failure to wean post CPB
-> bridge to LVAD
-> drug OD
-> sepsis
-> PE
-> cardiac or major vessel trauma
-> massive pulmonary haemorrhage
-> pulmonary trauma
-> acute anaphylaxis
- specific contraindications:
— aortic dissection and severe AR
Peripheral VA ECMO
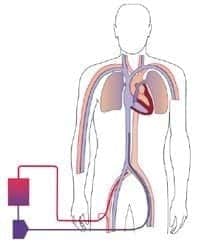
- back flow cannula used to prevent limb ischaemia
- oxygenated blood returned to aorta so lungs get little O2 rich blood -> may exacerbate lung ischaemia
- lower body receives better perfusion
Advantages
- good Q
- can create high oxygen tensions
Disadvantages
- relative lung ischaemia
- non-pulsatile blood flow
- possible poor perfusion of coronaries and cerebral vessels
- distal limb ischaemia
- risk of lung overventilation -> tissue alkalosis (monitor with ETCO2)
Central VA ECMO
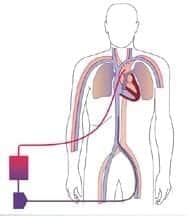
- cannula into the ascending aorta or subclavian artery (needs back flow cannula)
Advantages
- no preferential perfusion to lower body
- no possibility of hypoxic perfusion of cerebral vessels
- can use very large cannulae (high flows)
Disadvantages
- need sternotomy and tissue dissection
- predisposes to severe bleeding
CIRCUIT

- cannulae
- tubing
- pump
- membrane oxygenator and heat exchanger
- gas blender
Cannulae
- 15-23 Fr
- arterial
- venous
- back flow
- bicaval cannulae (available)
Tubing
- therapeutic anticoagulation required (ACT 180-200)
- all tubing is heparin bonded
- blood must be kept flowing
Pump
- centrifugal or roller
- patients on VV don’t require a pump as patients heart is working
Oxygenator
- large surface area
- integrated heat exchange
MANAGEMENT
Patient
- assessment if ECMO is no longer required:
VA
- PAC to assess pulmonary pressures
- TOE
- increasing pulsatility visible on arterial trace or increased MVO2
- turn down gas flow to oxygenator to assess respiratory function
VV
- CXR
- improved compliance
- reduced PaCO2
- turn off gas flow to oxygenator and increase ventilation
- minimise lung injury: ventilate to recruit but minimize VALI, measure ETCO2 to assess native lung recovery
ECMO
- optimise circuit function: pressure measurement before and after oxygenator
- optimise ‘gas exchange’: set flow to provide adequate O2 delivery and CO2 elimination, mixed venous saturation monitoring, adjust blood volume to influence MAP
- anticoagulation: Hb 120-130, ACT 140-180, platelets > 80
- removal of cannulae: surgical or compression depending on type of insertion
Mechanical ventilation initial seeting for VV-ECMO (consensus guidelines and expert opinion)
- Tidal volume: < 4 ml/kg predicted body weight
- Plateau pressure: < 25 cmH2O
- PEEP: 10-15 cmH2O
- FiO2: titrated to maintain sats > 85%
- RR: 4 to 6 breaths per minute
COMPLICATIONS
- clot formation
- haemolysis (plasma free Hb < 0.1g/L)
- suck down and kicking (due to vessel collapse around access cannulae)
- air embolism
- bleeding
- pump failure
- decannulation
- circuit rupture
- cardiac arrest
- oxygenator failure
- VA: left ventricular overdistension -> APO, cardiac damage, pulmonary haemorrhage, pulmonary infarction, aortic thrombosis, cardiac or cerebral hypoxia, CVA 15%
- VV: cardiac arrest -> perform CPR
SUMMARY
- well established in neonates
- adults and VV:
-> CESAR trial showed improved survival @ 6 months (63% vs 47%) in adults with severe acute respiratory failure
-> ANZ ECMO Influenza investigators showed a mortality rate of 21% (lower than previous published studies)
-> Systematic review on ECMO in H1N1 pandemic (Crit Care Med, 2010) found insufficient evidence for ECMO use among patients with H1N1
- adults and VA: little evidence so far
- CPR: used successfully in cardiac arrest
- retrieval: used successfully in retrieval medicine
-> established treatment
-> can decrease mortality
-> patient selection paramount
-> should be used in centres with appropriate expertise, experience and resources.
References and Links
CCC Ventilation Series
Modes: Adaptive Support Ventilation (ASV), Airway Pressure Release Ventilation (APRV), High Frequency Oscillation Ventilation (HFOV), High Frequency Ventilation (HFV), Modes of ventilation, Non-Invasive Ventilation (NIV), Spontaneous breathing and mechanical ventilation
Conditions: Acute Respiratory Distress Syndrome (ARDS), ARDS Definitions, ARDS Literature Summaries, Asthma, Bronchopleural Fistula, Burns, Oxygenation and Ventilation, COPD, Haemoptysis, Improving Oxygenation in ARDS, NIV and Asthma, NIV and the Critically Ill, Ventilator Induced Lung Injury (VILI), Volutrauma
Strategies: ARDSnet Ventilation, Open lung approach, Oxygen Saturation Targets, Protective Lung Ventilation, Recruitment manoeuvres in ARDS, Sedation pauses, Selective Lung Ventilation
Adjuncts: Adjunctive Respiratory Therapies, ECMO Overview, Heliox, Neuromuscular blockade in ARDS, Prone positioning and Mechanical Ventilation
Situations: Cuff leak, Difficulty weaning, High Airway Pressures, Post-Intubation Care, Post-intubation hypoxia
Troubleshooting: Autotriggering of the ventilator, High airway and alveolar pressures / pressure alarm, Ventilator Dyssynchrony
Investigation / Indices: A-a gradient, Capnography and waveforms, Electrical Impedance Tomography, Indices that predict difficult weaning, PaO2/FiO2 Ratio (PF), Transpulmonary pressure (TPP)
Extubation: Cuff Leak Test, Extubation Assessment in ED, Extubation Assessment in ICU, NIV for weaning, Post-Extubation Stridor, Spontaneous breathing trial, Unplanned extubation, Weaning from mechanical ventilation
Core Knowledge: Basics of Mechanical Ventilation, Driving Pressure, Dynamic pressure-volume loops, flow versus time graph, flow volume loops, Indications and complications, Intrinsic PEEP (autoPEEP), Oxygen Haemoglobin Dissociation Curve, Positive End Expiratory Pressure (PEEP), Pulmonary Mechanics, Pressure Vs Time Graph, Pressure vs Volume Loop, Setting up a ventilator, Ventilator waveform analysis, Volume vs time graph
Equipment: Capnography and CO2 Detector, Heat and Moisture Exchanger (HME), Ideal helicopter ventilator, Wet Circuit
MISC: Sedation in ICU, Ventilation literature summaries
Introduction to ICU Series
Introduction to ICU Series Landing Page
DAY TO DAY ICU: FASTHUG, ICU Ward Round, Clinical Examination, Communication in a Crisis, Documenting the ward round in ICU, Human Factors
AIRWAY: Bag Valve Mask Ventilation, Oropharyngeal Airway, Nasopharyngeal Airway, Endotracheal Tube (ETT), Tracheostomy Tubes
BREATHING: Positive End Expiratory Pressure (PEEP), High Flow Nasal Prongs (HFNP), Intubation and Mechanical Ventilation, Mechanical Ventilation Overview, Non-invasive Ventilation (NIV)
CIRCULATION: Arrhythmias, Atrial Fibrillation, ICU after Cardiac Surgery, Pacing Modes, ECMO, Shock
CNS: Brain Death, Delirium in the ICU, Examination of the Unconscious Patient, External-ventricular Drain (EVD), Sedation in the ICU
GASTROINTESTINAL: Enteral Nutrition vs Parenteral Nutrition, Intolerance to EN, Prokinetics, Stress Ulcer Prophylaxis (SUP), Ileus
GENITOURINARY: Acute Kidney Injury (AKI), CRRT Indications
HAEMATOLOGICAL: Anaemia, Blood Products, Massive Transfusion Protocol (MTP)
INFECTIOUS DISEASE: Antimicrobial Stewardship, Antimicrobial Quick Reference, Central Line Associated Bacterial Infection (CLABSI), Handwashing in ICU, Neutropenic Sepsis, Nosocomial Infections, Sepsis Overview
SPECIAL GROUPS IN ICU: Early Management of the Critically Ill Child, Paediatric Formulas, Paediatric Vital Signs, Pregnancy and ICU, Obesity, Elderly
FLUIDS AND ELECTROLYTES: Albumin vs 0.9% Saline, Assessing Fluid Status, Electrolyte Abnormalities, Hypertonic Saline
PHARMACOLOGY: Drug Infusion Doses, Summary of Vasopressors, Prokinetics, Steroid Conversion, GI Drug Absorption in Critical Illness
PROCEDURES: Arterial line, CVC, Intercostal Catheter (ICC), Intraosseous Needle, Underwater seal drain, Naso- and Orogastric Tubes (NGT/OGT), Rapid Infusion Catheter (RIC)
INVESTIGATIONS: ABG Interpretation, Echo in ICU, CXR in ICU, Routine daily CXR, FBC, TEG/ROTEM, US in Critical Care
ICU MONITORING: NIBP vs Arterial line, Arterial Line Pressure Transduction, Cardiac Output, Central Venous Pressure (CVP), CO2 / Capnography, Pulmonary Artery Catheter (PAC / Swan-Ganz), Pulse Oximeter
LITFL
- CCC – Everything ECMO
- CCC – ECMO troubleshooting
- CCC – Pharmacokinetics and ECMO
Journal articles
- Bartlett RH, Gattinoni L. Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva Anestesiol. 2010 Jul;76(7):534-40. PubMed PMID: 20613694. [Free Full Text]
- Baud FJ, Megarbane B, Deye N, Leprince P. Clinical review: aggressive management and extracorporeal support for drug-induced cardiotoxicity. Crit Care. 2007;11(2):207. Review. PubMed PMID: 17367544; PubMed Central PMCID: PMC2206443.
- Chauhan S, Subin S. Extracorporeal membrane oxygenation, an anesthesiologist’s perspective: physiology and principles. Part 1. Ann Card Anaesth. 2011 Sep-Dec;14(3):218-29. doi: 10.4103/0971-9784.84030. Review. PubMed PMID: 21860197. [Free Fulltext]
- Chauhan S, Subin S. Extracorporeal membrane oxygenation–an anaesthesiologist’s perspective–Part II: clinical and technical consideration. Ann Card Anaesth. 2012 Jan-Mar;15(1):69-82. doi: 10.4103/0971-9784.91485. Review. PubMed PMID: 22234027. [Free Fulltext]
- Ensminger SL, et al. The role of extracorporeal mechanical assists. (ECLS et al.) Applied Cardiopulmonary Pathophysiology 16: 192-201, 2012 [Free Fulltext]
- Gattinoni L, Carlesso E, Langer T. Clinical review: Extracorporeal membrane oxygenation. Crit Care. 2011;15(6):243. doi: 10.1186/cc10490. Epub 2011 Dec 8. Review. PubMed PMID: 22188792; PubMed Central PMCID: PMC3388693.
- Lindstrom SJ, Pellegrino VA, Butt WW. Extracorporeal membrane oxygenation. Med J Aust. 2009 Aug 3;191(3):178-82. Review. PubMed PMID: 19645652. [Free Fulltext]
- Pellegrino VA, Davies AR. CESAR: deliverance or just the beginning? Crit Care Resusc. 2010 Jun;12(2):75-7. PubMed PMID: 20513213. [Free Fulltext]
- Schmidt M, et al. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care 2014;18:203.
FOAM and web resources
- ECMO.ICU – Alfred ICU ECMO guidelines available as a free-to-access website.
- INTENSIVE – Intro to ECMO for New ICU Staff (Ken Hoffman, 2023)
- INTENSIVE – ECMO (collated ECMO resources)

Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC
