Killer ECG Patterns: Part 1
Peer reviewed with thanks by Dr Stephen Smith of Dr Smith’s ECG Blog
The average Emergency Clinician:
- Performs around 100 tasks per hour
- Gets interrupted every 6 minutes
In many Emergency Departments, senior doctors are handed an ECG every 10-15 minutes. Typically, these ECGs come from ATS category 2 patients that have been triaged but not yet seen by a doctor. When you are busy with your own workload, it can be tempting to quickly “sign off” on the ECG, deferring further medical assessment until there is a doctor available to see the patient.
PART ONE: “Deadly Diagnoses Not To Miss” (the Non-ischaemic version…)
PART TWO: “Deadly Diagnoses Not To Miss” (the Occlusion version…)
Recognition of any of these killer ECG patterns should prompt expedited medical assessment and treatment.
ECG 1
25-year-old with exertional dizziness.
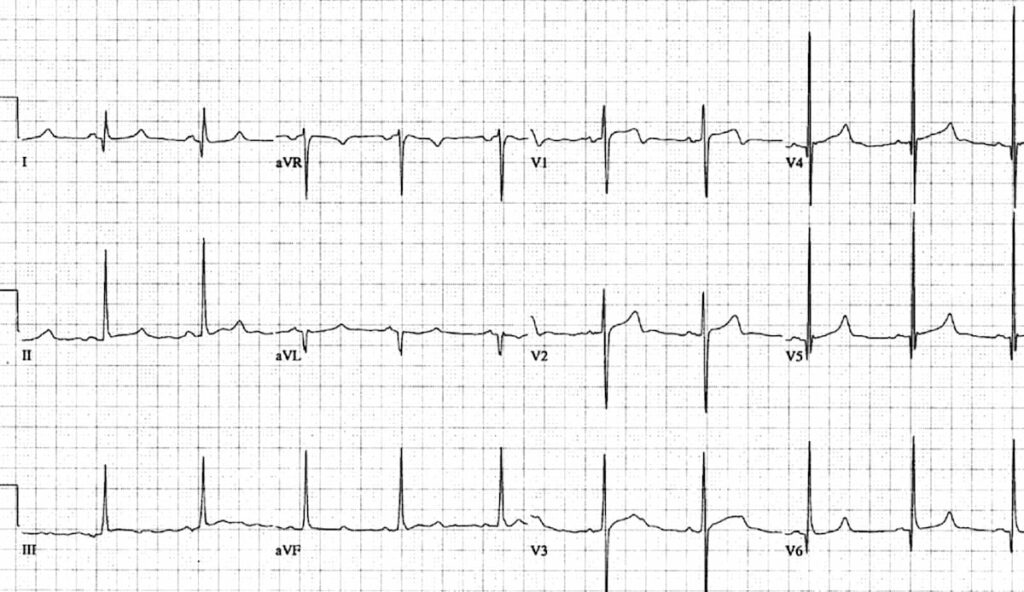
Q1. What are the main abnormalities present on this ECG?
Reveal Answer
- Voltage criteria for left ventricular hypertrophy (LVH) — S wave in V1 + R wave in V5 > 35mm (note there are many different ways of measuring voltage criteria for LVH)
- Deep, narrow Q waves in lateral leads I, aVL, V5-6
Q2. What is the likely diagnosis?
Reveal Answer
Hypertrophic Cardiomyopathy (HCM)
HCM is an inherited cardiac disorder and is the number one cause of sudden cardiac death in young people. Genetic mutations cause left ventricular hypertrophy in the absence of any inciting stimulus. Both the degree and distribution of LVH is variable, and can range from mild (13-15mm) to extreme myocardial thickening (30mm+).
Clinical features depend on the location of hypertrophy.
The most commonly observed pattern is asymmetrical thickening of the interventricular septum (IVS). This predisposes to obstruction of the left ventricular outflow tract, causing exertional presyncope or syncope in 25% of cases.
Left ventricular diastolic dysfunction can cause prolonged apical contraction and impaired LV filling, leading to anginal symptoms (see below video). Lastly, disorganised LV architecture predisposes to abnormal conduction and fatal arrhythmias.
Here is a quick 2-minute video describing the bedside echo features of HCM:
ECG features in HCM
The ECG manifestations of HCM reflect conduction through hypertrophied tissue, particularly a thickened IVS (causing dagger Q waves):
- Left ventricular hypertrophy with increased precordial voltages and non-specific ST segment and T-wave abnormalities
- Deep, narrow (“dagger-like”) Q waves in lateral (I, aVL, V5-6) +/- inferior (II, III, aVF) leads
Left atrial enlargement is common in severe disease and is due to compensatory hypertrophy in response to LV diastolic dysfunction.
Q3. What should be done next?
Reveal Answer
Patients with an ECG suggestive of HCM who are symptomatic require immediate admission for cardiac monitoring, formal echo, and cardiac MRI to confirm the diagnosis and degree of severity of disease.
Those who are asymptomatic can usually have the above performed as an outpatient.
Note that 5% of patients with HCM will have a normal ECG. If there is clinical suspicion based on symptoms, then the above investigations should be performed.
ECG 2
17-year-old noticed “high” heart rate on Apple watch.
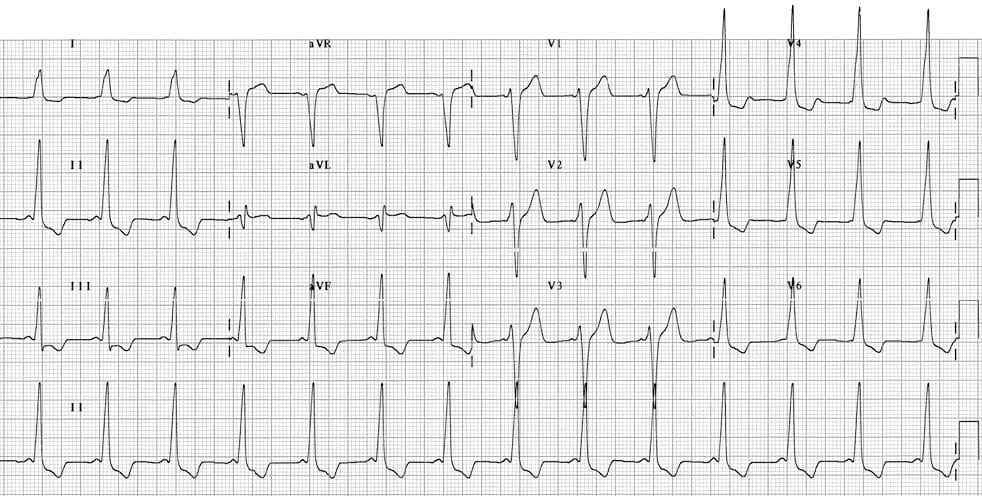
Q1. What are the main abnormalities present on this ECG?
Reveal Answer
On first glance this ECG may appear to represent left ventricular hypertrophy, as we see large voltage QRS complexes and ST/T wave abnormalities in lateral leads.
However, on closer inspection, other abnormalities are present:
- Very short PR interval (< 120ms)
- Broad QRS complexes with a slurred upstroke to the QRS complexes – the delta wave
Q2. What is the likely diagnosis?
Reveal Answer
Wolff-Parkinson-White Syndrome (WPW)
WPW refers to the presence of a congenital accessory pathway (between atria and ventricles) and episodes of tachyarrhythmias.
An accessory pathway can conduct impulses either anterograde (towards the ventricle), retrograde (away from the ventricle), or in both directions. The majority of pathways allow conduction in both directions.
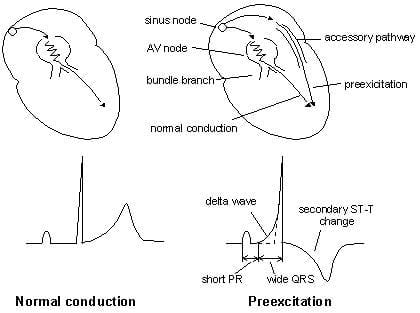
ECG features of WPW
- PR interval < 120ms
- Delta wave: slurring slow rise of initial portion of the QRS
- QRS prolongation > 110ms
- ST-segment and T-wave discordant changes – i.e. in the opposite direction to the major component of the QRS complex
Note that tall R waves may mimic ventricular hypertrophy but are simply a result of abnormal depolarisation through an accessory pathway. When there is abnormal depolarisation there should be abnormal repolarisation — hence the presence of T-wave inversion and ST elevation and/or depression, which can often mimic Occlusion Myocardial Infarction (OMI).
There are two types of precordial patterns seen on the sinus rhythm ECG – type A and type B. The type A pattern is associated with a left-sided accessory pathway and manifests a dominant R wave in V1 that may mimic right ventricular hypertrophy.
Our patient above has a type B pattern – a dominant S wave in V1 indicates a right-sided accessory pathway. Note how this could be confused for LVH.
The presence of an accessory pathway predisposes patients to tachyarrhythmia formation. This can be facilitated by:
- Formation of a reentry circuit involving the accessory pathway, termed atrioventricular reentry tachycardias (AVRT)
- Direct conduction from atria to ventricles via the accessory pathway, bypassing the AV node, seen with atrial fibrillation of atrial flutter in conjunction with WPW
15% of cases of WPW exhibit retrograde conduction only – as such, they are a “concealed pathway” on the ECG during sinus rhythm. Manifestations of disease will only be evident when tachyarrhythmias arise.
Q3. What should be done next?
Reveal Answer
Identification of a short PR interval and a delta wave on the ECG only confirms the presence of a WPW pattern. The diagnosis of WPW syndrome is made when there is a history or subsequent development of an arrhythmia.
The preferred long-term approach for patients with an accessory pathway is ablation. In the acute setting, knowledge of the presence of an accessory pathway is critical as some standard therapies for tachyarrhythmias may cause clinical deterioration in patients with WPW.
You can read more about WPW and management of specific tachyarrhythmia complications here.
ECG 3
75-year old with atypical chest pain.
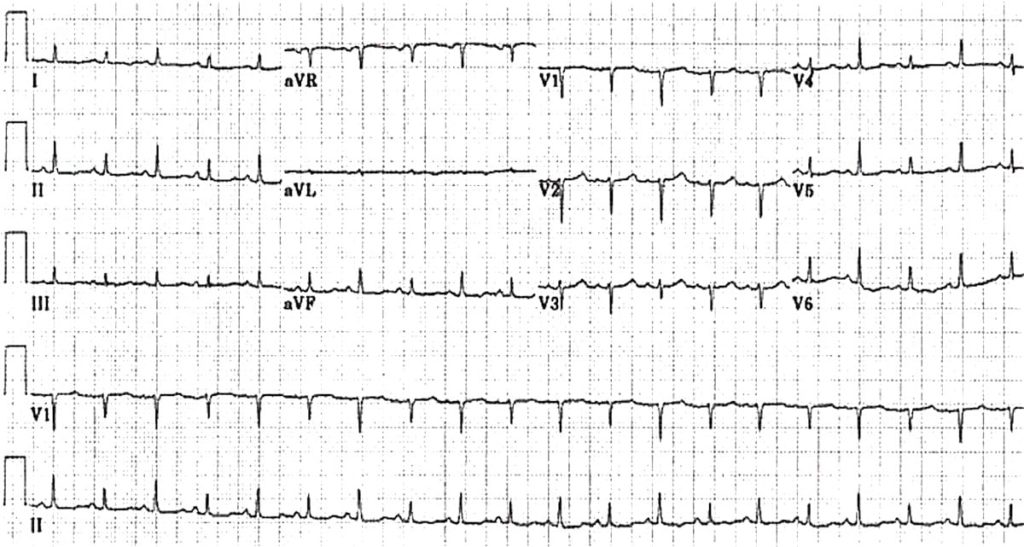
Q1. What are the main abnormalities present on this ECG?
Reveal Answer
- Sinus tachycardia ~120 bpm
- Low QRS voltages
- Electrical alternans (= alternating tall and short QRS complexes)
Q2. What is the likely diagnosis?
Reveal Answer
The combination of low QRS voltages and electrical alternans is highly suggestive of massive pericardial effusion. The addition of sinus tachycardia is concerning for pericardial tamponade.
Low voltage generally refers to QRS complex amplitude:
- < 5 mm (0.5 mV) in the limb leads
- < 10 mm (1 mV) in the precordial leads
However, these criteria are not 100% sensitive for the presence of large pericardial effusion — patients can have a significant effusion without reduction in QRS amplitude.
Electrical alternans refers to a beat-to-beat variation in the QRS complex height, with alternating taller and shorter QRS complexes. It is thought to be due to the heart swinging backwards and forwards within a fluid-filled pericardial sac.
Sinus tachycardia occurs as a compensatory phenomenon in cardiac tamponade to maintain cardiac output in the face of diminishing stroke volume. It is of course non-specific, and may also be due to pain, anxiety, shortness of breath or other causes.
Q3. What should be done next?
Reveal Answer
This patient needs an immediate bedside echo to confirm the presence of a pericardial effusion and to assess for signs of tamponade. Here is a nice example from James Rippey of what we might see in this patient.
Further management will depend on these findings and the clinical picture. Patients with tamponade will usually have a degree of haemodynamic instability. In the face of haemodynamic compromise, emergency pericardiocentesis or ED thoracotomy may be required depending on the cause.
Note that the presence of tamponade relates more to the rapidity of onset rather than the size of effusion. A patient with an acute aortic dissection can have impaired right ventricular filling as a result of a 200ml effusion, whereas an oncology patient with a chronic 800ml effusion may have no haemodynamic instability. This relates to compliance of the pericardium over time.
ECG 4
30-year old with palpitations.
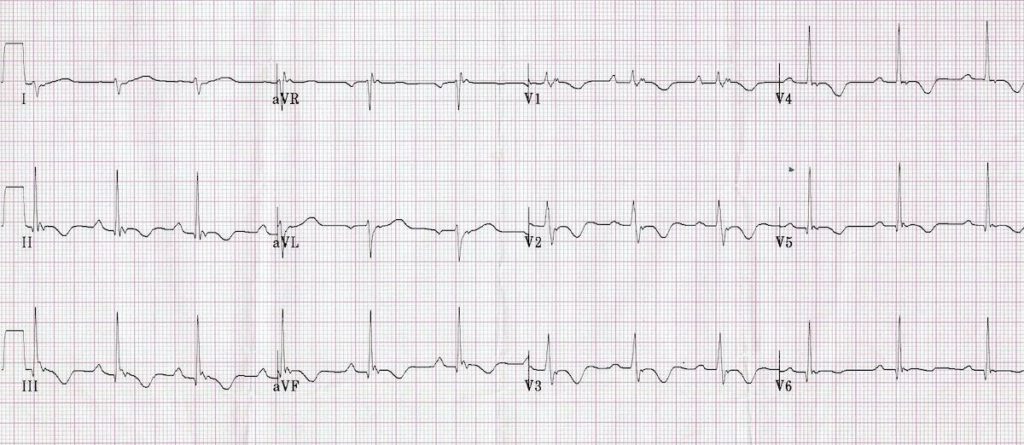
Q1. What are the main abnormalities present on this ECG?
Reveal Answer
- Right axis deviation
- Dominant R wave in V1
- Widespread T-wave inversion in inferior (II, III, aVF) and precordial leads (V1-6)
- Localised subtle widening of QRS complexes in V1-3
- A small blip following each QRS complex, best seen in V1 and the inferior leads — this is known as an Epsilon wave
Q2. What is the significance of these ECG changes?
Reveal Answer
- Right axis deviation and a dominant R wave in V1 are signs of Right Ventricular Hypertrophy (RVH)
- Concurrent T-wave inversion in inferior and right precordial (V1-3) reflect a Right Ventricular Strain pattern, and are another sign of RVH
- These findings, along with localised QRS widening in V1-3 and the presence of Epsilon waves, are highly suggestive of Arrhythmogenic Right Ventricular Dysplasia (ARVD)
Arrhythmogenic Right Ventricular Dysplasia
- Autosomal dominant genetic disorder of myocardium in which there is fatty infiltration of the right ventricular free wall, predisposing to paroxysmal ventricular arrhythmias, sudden cardiac death, and biventricular failure
- Second most common cause of sudden cardiac death in young people (after hypertrophic cardiomyopathy), accounting for up to 10% of sudden cardiac deaths in patients < 65 years of age
Clinical Features
- ARVD causes symptoms due to frequent ventricular ectopic beats or sustained ventricular tachycardia (with LBBB morphology) — patients typically present with palpitations, syncope or cardiac arrest precipitated by exercise
- The first presenting symptom may be sudden cardiac death
Q3. What should be done next?
Reveal Answer
- Echocardiography is the first-line investigation, and may demonstrate a dilated, hypokinetic right ventricle with prominent apical trabeculae and dilatation of the RV outflow tract
- The imaging modality of choice in many centres is cardiovascular MRI, which can accurately demonstrate structural and functional features of ARVD. These include fibrofatty infiltration and thinning of the RV myocardium, RV aneurysms, RV dilatation, regional wall motion abnormalities and global systolic dysfunction
Read more about the ECG features of ARVD here.
ECG 5
60-year old with shortness of breath.
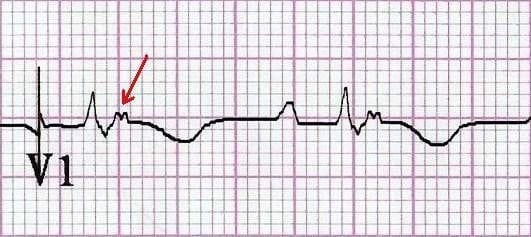
Q1. What are the main abnormalities present on this ECG?
Reveal Answer
- Severe bradycardia (HR ~ 30 bpm)
- Flattening, broadening and near-disappearance of P waves (still barely visible in V1-3)
- Prolongation of the PR interval
- Broad QRS complexes (~120 ms)
- Symmetrically peaked T waves in V2-5
Q2. What is the likely diagnosis?
Reveal Answer
This patient presented to ED with pulmonary oedema after missing several dialysis sessions. Shortly after this ECG was taken, her HR dropped to < 10 bpm with no palpable pulse requiring a brief period of CPR. ROSC was rapidly achieved following empirical administration of IV calcium gluconate. Bedside VBG taken on arrival later revealed a K+ of 7.9.
NB: The differential diagnosis for this patient with severe bradycardia and 1st degree AV block would include beta-blocker and calcium-channel blocker toxicity.
Q3. What are the other ECG manifestations of this condition?
Reveal Answer
ECG features of hyperkalaemia
- Peaked, tented T waves
- P wave widening/flattening, PR prolongation
- Bradyarrhythmias: sinus bradycardia, high-grade AV block with slow junctional and ventricular escape rhythms, slow AF
- Conduction blocks (bundle branch block, fascicular blocks)
- QRS widening with bizarre QRS morphology
Worsening hyperkalaemia (> 8.0-9.0 mmol/L) may precipitate development of a sine wave appearance (pre-terminal rhythm); ventricular fibrillation; PEA with bizarre, wide complex rhythm; and eventually asystole.
Serum potassium level does not always correlate closely with ECG changes. Patients with a relatively normal ECG can suffer sudden hyperkalaemia cardiac arrest. Any patient who has had a bradycardia PEA arrest has hyperkalaemia until proven otherwise.
The push-pull effect
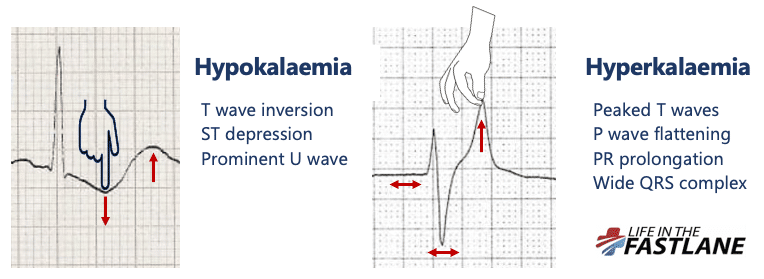
- Hyperkalaemia creates the illusion that the T wave is “pulled upwards”, creating tall “tented” T waves, and stretching the remainder of the ECG to cause P wave flattening, PR prolongation, and QRS widening
An easy way to remember the usual order of ECG changes seen is by following the ECG trace logically – effects most commonly begin on the T wave and move forwards to the P wave / PR interval, and subsequently to the QRS complex with QRS widening and conduction blocks.
Read more about the ECG manifestations of hyperkalaemia.
ECG 6
30-year old with drowsiness.
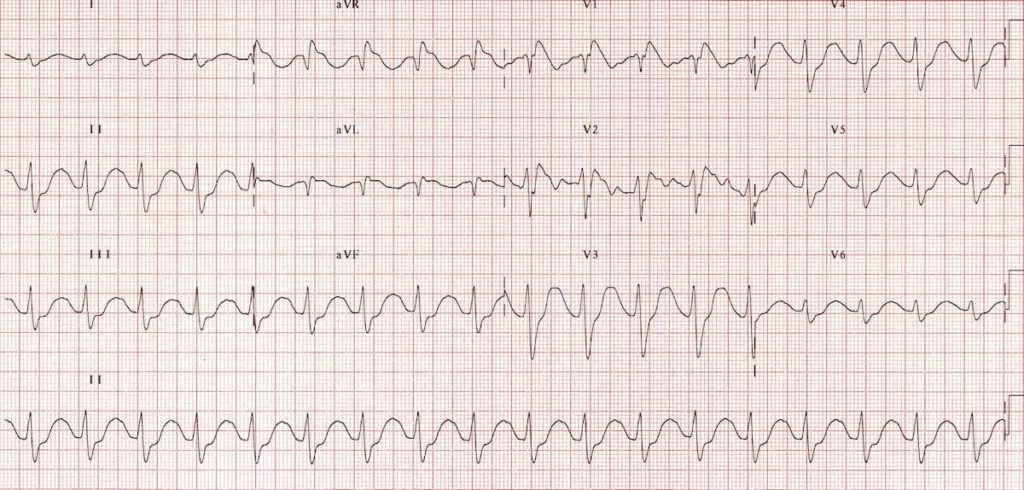
Q1. What are the main abnormalities present on this ECG?
Reveal Answer
- Sinus tachycardia ~110 bpm — P waves are visible in V2
- Borderline 1st degree AV block
- Broad QRS complexes (120 ms, or 3 small squares)
- Dominant R’ wave in lead aVR
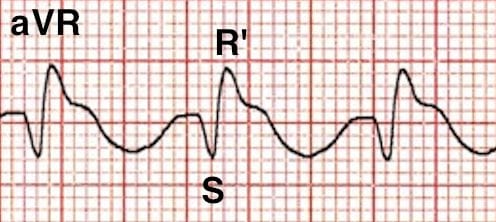
Q2. What is the likely diagnosis?
Reveal Answer
The combination of broad QRS complexes and a dominant R’ wave in aVR is strongly suggestive of poisoning with a sodium-channel blocking agent. The most commonly encountered examples include:
- Tricyclic antidepressants (TCAs)
- Propranolol
- Carbamazepine
- Type IA and IC antiarrhythmics (quinidine, procainamide, flecainide)
- Local anaesthetics
- Cocaine
These agents can cause seizures and cardiotoxicity (hypotension, broad-complex tachycardia) in overdose.
TCAs also cause tachycardia due to their anticholinergic effects, whilst propranolol can cause bradycardia due to beta-blocking effects.
Other mimics that can cause this ECG pattern include hyperkalaemia and Brugada Syndrome. Always consider these differentials and correlate with the clinical context.
ECG Features of Sodium Channel Blockade
- Intraventricular conduction delay: QRS > 100 ms in lead II
- Terminal R wave > 3 mm in aVR or R/S ratio > 0.7 in aVR
The right-sided intraventricular conducting system is more susceptible to the effects of sodium channel blockade — this causes terminal right axis deviation of the QRS complex, which manifests as a dominant R’ wave in aVR.
The degree of QRS prolongation in TCA overdose correlates with the degree of clinical toxicity:
- QRS width > 100 ms is predictive of seizures
- QRS width > 160 ms is predictive of cardiotoxicity (e.g. broad-complex dysrhythmias, hypotension)
Learn more about the effects of sodium channel blocking agents on the ECG, and specific management priorities, here.
ECG 7
25-year old with collapse, apparently alcohol intoxicated.
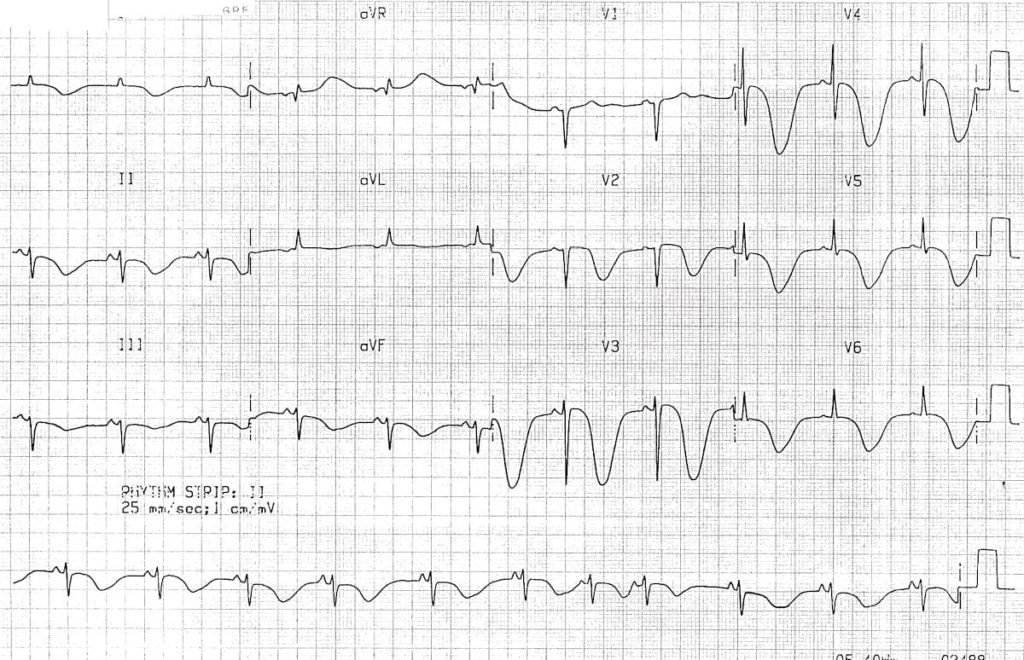
Q1. What are the main abnormalities present on this ECG?
Reveal Answer
- Widespread, giant T wave inversions
- Grossly prolonged QT interval (~ 600ms)
Q2. What is the likely diagnosis?
Reveal Answer
This ECG pattern is characteristic of raised intracranial pressure and is classically seen in the context of massive intracranial haemorrhage, particularly:
- Spontaneous subarachnoid haemorrhage
- Haemorrhagic stroke / intraparenchymal haemorrhage
Similar ECG patterns have also been reported in patients with raised ICP due to:
- Large-territory ischaemic stroke causing cerebral oedema (e.g. MCA occlusion)
- Traumatic brain injury
The main differential diagnosis for widespread “giant” T-wave inversion such as this is apical hypertrophic cardiomyopathy (HCM). Although myocardial ischaemia and electrolyte abnormalities can cause widespread T-wave inversion and QT prolongation, neither condition would cause the gigantic “cerebral T waves” seen here.
Q3. What should be done next?
Reveal Answer
Hmmm…
Obviously this is an artificial situation. This patient is likely to be deeply comatose on arrival to hospital — potentially with focal neurology — necessitating early airway intervention, measures to reduce ICP, and expedited neuroimaging to confirm the diagnosis. The ECG should not be relied upon to make this diagnosis!
In any case, their CT scan might look something like this…

Further Learning
ECG 8
40-year old with syncope.
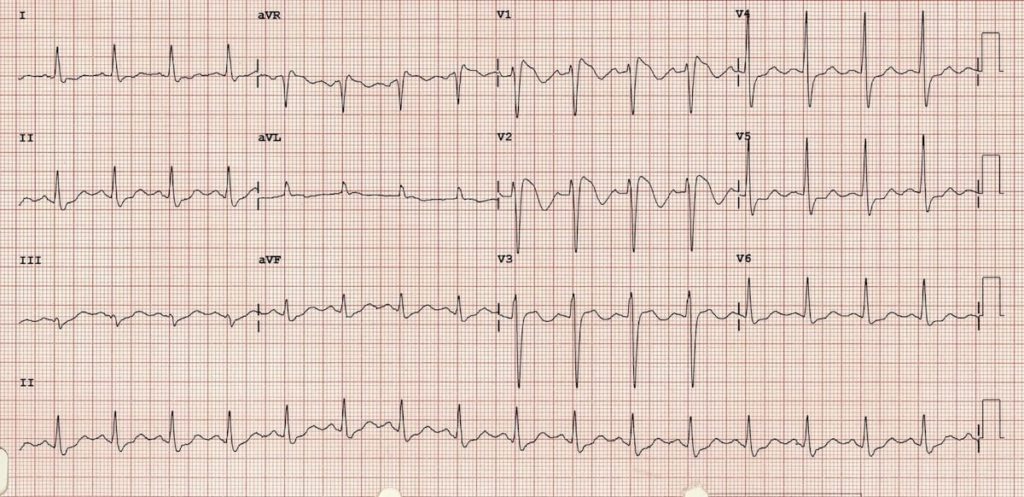
Q1. What are the main abnormalities present on this ECG?
Reveal Answer
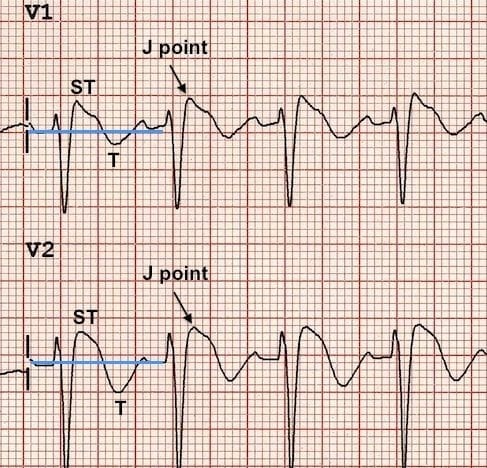
At first glance, this ECG may appear normal with no evidence of arrhythmia or AV block to explain the syncopal episode. However, on closer inspection there are some localised abnormalities in the right precordial leads V1 and V2:
- RBBB-like pattern with secondary R’ wave following the QRS complex
- ST elevation at the J point > 2mm with a “coved” morphology — the ST segment slopes diagonally downwards from the J point, with a slight upward convexity best appreciated in V2
- Associated T wave inversion
Q2. What is the likely diagnosis?
Reveal Answer
In a patient presenting with syncope, this ECG pattern is diagnostic of Brugada Syndrome.
Brugada syndrome is:
- An arrhythmogenic sodium channelopathy caused by a mutation in the cardiac sodium gene — this can be inherited or spontaneous
- Most common in South East Asian males, with presentation around age 40
- Associated with increased risk of paroxysmal ventricular arrhythmias (polymorphic VT, VF) and sudden cardiac death
Patients present with:
- Sudden cardiac death
- Symptomatic ventricular arrhythmias (paroxysmal syncope, seizure-like events, nocturnal agonal respirations)
- Asymptomatic – after family screening or incidental finding on ECG recording
The only effective treatment is insertion of an implantable cardioverter-defibrillator (ICD).
What are the ECG features of Brugada Syndrome?
Reveal answer
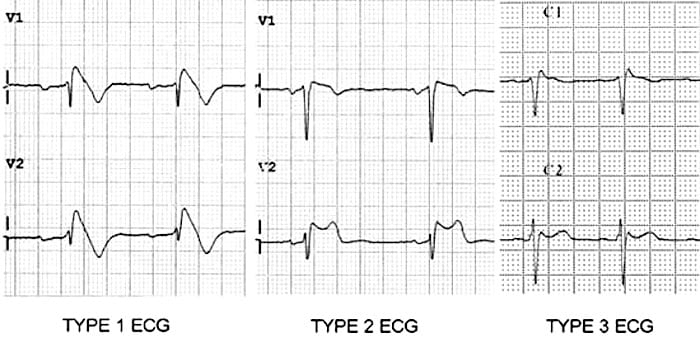
There are three ECG patterns associated with Brugada syndrome, of which only the type 1 ECG is diagnostic. Changes need to occur in at least 2 of the right precordial leads (V1-3).
Brugada ECG patterns
- Type 1 = “Coved” ST elevation > 2mm at the J-point, followed by an inverted T wave
- Type 2 = “Saddleback” ST segments with > 2mm J-point elevation, > 1mm ST elevation and a positive or biphasic T wave
- Type 3 = Coved or saddleback ST elevation < 1mm
The ECG pattern may vary over time — patients with symptomatic Brugada syndrome may have a non-diagnostic ECG at the time of assessment (e.g. Type 2 or 3 pattern; even a normal ECG). A diagnostic ECG may be produced in these patients by administration of a sodium-channel blocking agent, typically a class I antiarrhythmic such as flecainide. Interestingly, fever has also been shown to unmask the type 1 ECG pattern and may precipitate ventricular arrhythmias.
Tip – the sensitivity of the ECG for detecting Brugada syndrome may be increased by placing leads V1 and V2 in the second (rather than the fourth) intercostal space.
How do I make the diagnosis of Brugada Syndrome?
Reveal answer
Diagnosis of Brugada syndrome requires both:
- Diagnostic (Type 1) ECG pattern – either spontaneously, or during pharmacological challenge with class I antiarrhythmics
- At least one clinical criterion
Clinical Criteria
- Positive family history: Sudden cardiac death in family member aged < 45; type 1 ECG pattern in family member
- Arrhythmia-related symptoms: Cardiac syncope; seizure-like events; nocturnal agonal respirations
- Documented ventricular arrhythmias: Polymorphic ventricular tachycardia (PVT); ventricular fibrillation (VF)
Diagnostic difficulties arise when faced with patients that do not meet the above criteria, for example:
- Idiopathic type 1 ECG
- Type 2 or 3 ECG pattern
How do I manage my patient with a Brugada ECG pattern?
Reveal answer
Symptomatic patients with a type 1 ECG pattern
These patients are admitted for cardiac monitoring and ICD insertion.
Idiopathic type 1 ECG
This refers to patients with a type 1 ECG pattern but no positive clinical criteria. It is unclear whether these patients are at increased risk of sudden cardiac death. Further risk stratification with electrophysiological study (EPS) is recommended.
Patients who develop PVT/VF during EPS are classified as higher risk and referred for ICD insertion. Patients with a negative EPS are followed up closely by an electrophysiologist, but do not receive an ICD unless they subsequently develop symptoms.
Type 2 and 3 ECG patterns
As these ECG patterns are non-specific, the extent to which these patients are worked up depends on the individual merits of the case and the degree of clinical suspicion for Brugada syndrome.
Patients with a compelling story for Brugada syndrome (e.g. recurrent cardiovascular syncope, resuscitated cardiac arrest) should be admitted for monitoring and further diagnostic workup — e.g. with flecainide challenge. Patients with asymptomatic type 2 and 3 patterns may require further investigation if there is a family history of Brugada syndrome.
Q3. What should be done next?
Reveal Answer
Our patient is symptomatic with a type 1 ECG pattern — he requires immediate admission for cardiac monitoring and ICD insertion.
Conclusions
- ECG interpretation is essentially pattern recognition
- Calibrate your pattern recognition skills by looking at as many ECGs as you can. Further calibrate your skills by exploring some of the excellent ECG blogs out there, and by following up your own patients after confirmatory testing (e.g. angiography) to see whether your predictions about their ECG were correct
- Remember that the ECG in isolation is rarely sensitive or specific enough to make a firm diagnosis — all of these killer ECG patterns require clinical correlation and/or confirmatory investigations
Here is a quick summary sheet — feel free to share with your colleagues!
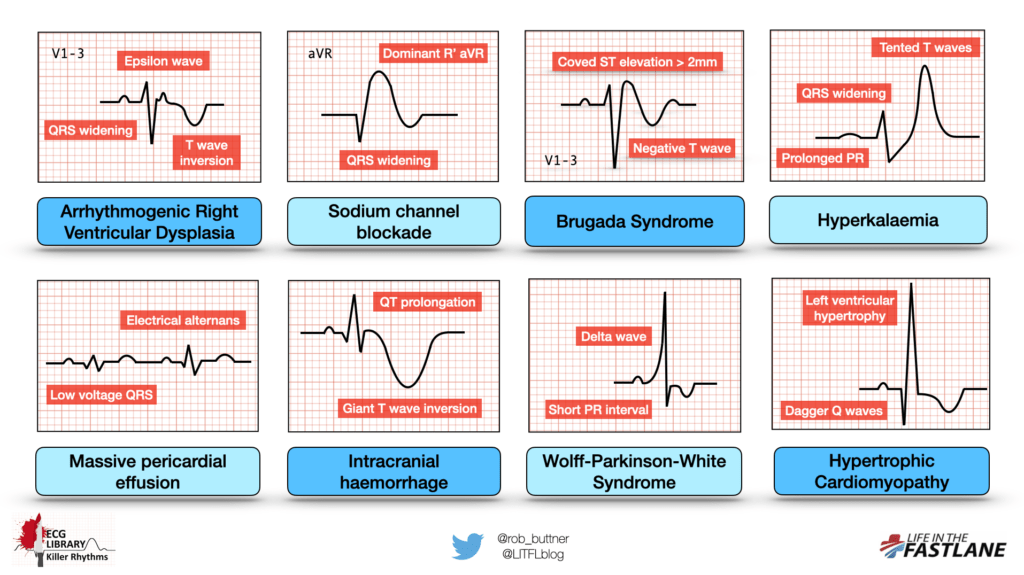
Advanced Reading
- Burns E, Buttner R. Killer ECG Patterns: Part 1 – Non-ischaemic. LITFL
- Buttner R, Aslanger E. Killer ECG Patterns: Part 2 – Occlusion. LITFL
Online
- Wiesbauer F, Kühn P. ECG Mastery: Yellow Belt online course. Understand ECG basics. Medmastery
- Wiesbauer F, Kühn P. ECG Mastery: Blue Belt online course: Become an ECG expert. Medmastery
- Kühn P, Houghton A. ECG Mastery: Black Belt Workshop. Advanced ECG interpretation. Medmastery
- Rawshani A. Clinical ECG Interpretation ECG Waves
- Smith SW. Dr Smith’s ECG blog.
- Wiesbauer F. Little Black Book of ECG Secrets. Medmastery PDF
Textbooks
- Zimmerman FH. ECG Core Curriculum. 2023
- Mattu A, Berberian J, Brady WJ. Emergency ECGs: Case-Based Review and Interpretations, 2022
- Straus DG, Schocken DD. Marriott’s Practical Electrocardiography 13e, 2021
- Brady WJ, Lipinski MJ et al. Electrocardiogram in Clinical Medicine. 1e, 2020
- Mattu A, Tabas JA, Brady WJ. Electrocardiography in Emergency, Acute, and Critical Care. 2e, 2019
- Hampton J, Adlam D. The ECG Made Practical 7e, 2019
- Kühn P, Lang C, Wiesbauer F. ECG Mastery: The Simplest Way to Learn the ECG. 2015
- Grauer K. ECG Pocket Brain (Expanded) 6e, 2014
- Surawicz B, Knilans T. Chou’s Electrocardiography in Clinical Practice: Adult and Pediatric 6e, 2008
- Chan TC. ECG in Emergency Medicine and Acute Care 1e, 2004
LITFL Further Reading
- ECG Library Basics – Waves, Intervals, Segments and Clinical Interpretation
- ECG A to Z by diagnosis – ECG interpretation in clinical context
- ECG Exigency and Cardiovascular Curveball – ECG Clinical Cases
- 100 ECG Quiz – Self-assessment tool for examination practice
- ECG Reference SITES and BOOKS – the best of the rest
ECG LIBRARY
Emergency Physician in Prehospital and Retrieval Medicine in Sydney, Australia. He has a passion for ECG interpretation and medical education | ECG Library |
MBBS DDU (Emergency) CCPU. Adult/Paediatric Emergency Medicine Advanced Trainee in Melbourne, Australia. Special interests in diagnostic and procedural ultrasound, medical education, and ECG interpretation. Co-creator of the LITFL ECG Library. Twitter: @rob_buttner


Awesome cases, great reviews.
I am happy to say that I got all the cases correct, only because I follow what Ed and Robert post.
Thanks guys!
thank you for the great summary! look forward to part 2!