Etomidate
CLASS
- General anaesthetic / induction agent
- Carboxylated imidazole derivative
PHYSICOCHEMICAL PROPERTIES
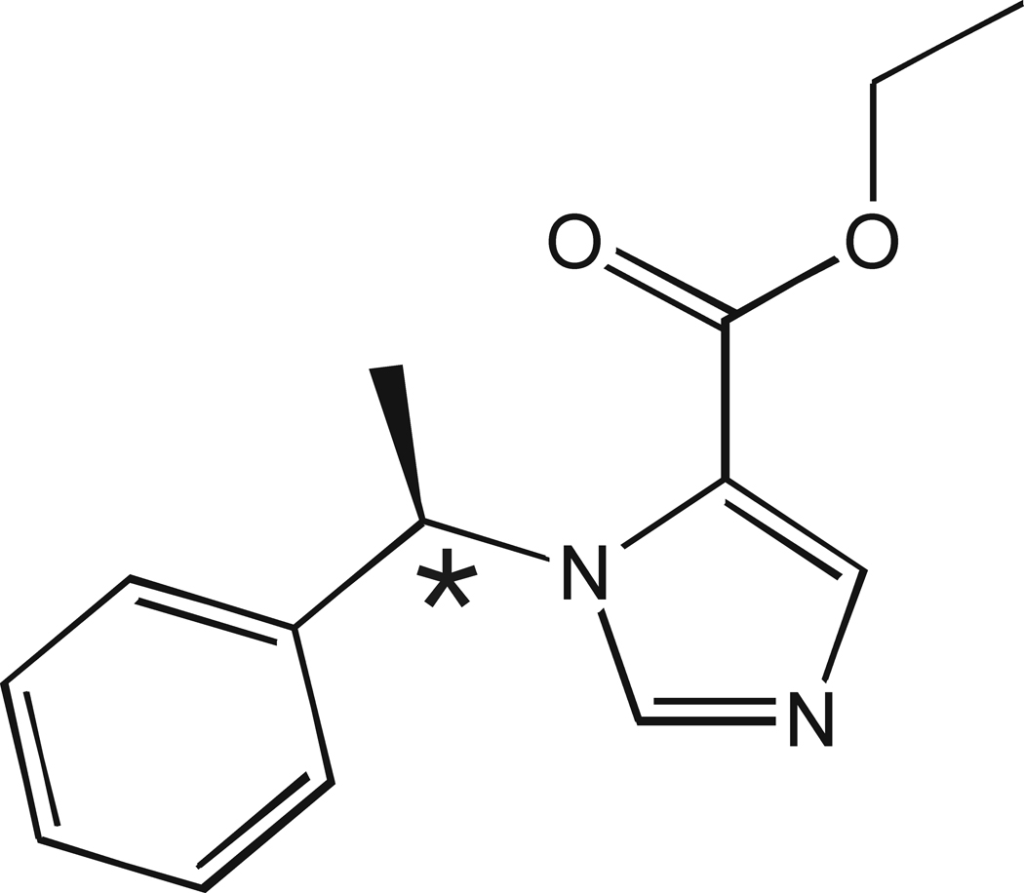
- 1-(1-ethylphenyl)imidazole-5-ethyl ester
- It has a chiral center (* in figure) – the R(+) isomer of etomidate is ten times more potent than its S(−) isomer at potentiating gamma-aminobutyric acid (GABA) type A receptor activity
- weak base with a pKa of 4.2
MECHANISM OF ACTION
- Acts primarily as a positive allosteric potentiator of the GABA-A receptor (increases the effect of GABA) by stabilising the open state of the channel.
- In supra-clinical doses also has a direct agonist effect on GABA-A receptors.
- Anatomic Locations:
- Enhances GABAergic inhibition in the reticular activating system of the brainstem, leading to sedation and hypnosis.
- Second Messenger Pathways:
- Increases chloride ion influx through GABA-A receptor channels, hyperpolarizing neurons and inhibiting action potentials.
- Pharmacodynamic Effects:
- Sedation and Hypnosis: Rapid onset of unconsciousness.
- Minimal Cardiovascular Effects: Maintains hemodynamic stability.
- Adrenal Suppression: Inhibits 11β-hydroxylase, reducing cortisol synthesis. This effect has much higher potency than the sedative/ hypnotic effect.
- No analgesic effect.
- Duration of action:
- 3-8 minutes with standard induction doses, due to rapid redistribution to muscle and later to fat.
- Duration of action increases linearly with increasing dose – each 0.1 mg/kg IV provides about 100 seconds of unconsciousness.
PHARMACEUTICS
- Formulations:
- Intravenous (IV) injection
- Concentrations: Typically available as 2 mg/mL solution in 35% propylene glycol due to poor water solubility
- Aqueous solution has pH 6.9
- Lipid emulsion form is less irritant and faster onset.
DOSE
- IV Bolus: 0.2-0.6 mg/kg over 30-60 seconds for induction of anesthesia.
- Continuous Infusion: 0.04-0.05 mg/kg/hr for adrenal suppression to treat severe Cushing syndrome (off-label).
INDICATION
- Induction of general anesthesia.
- Rapid sequence intubation when haemodynamic stability is a priority
- Procedural sedation for very brief procedures
- Emergency management of severe Cushing syndrome (“Cushing crisis”) (off-label use).
CONTRA-INDICATIONS
Absolute
- Hypersensitivity
- Porphyria
Precautions
- Adrenal insufficiency
- Cirrhosis and oesophageal varices – volume of distribution and elimination half-life are doubled.
DRUG-DRUG INTERACTIONS
- Alcohol, opioids, sedatives, sodium oxybate, MAO inhibitors – additive sedative effect
- Verapamil, reserpine – additive cardiovascular depression
- GLP-1 agonists – additive nausea/vomiting risk due to slow gastric emptying
ADVERSE EFFECTS
- Cardiovascular:
- Usually minimal changes in HR and BP
- Minimal decrease in SVR
- Negligible cardiodepressant effect at induction doses (e.g. <0.6 mg/kg).
- does not alter sympathetic or baroreceptor reflexes
- Hypotension/ cardiovascular collapse in the critically ill, in the elderly, if hypovolaemia present, or combined with other agents
- Neurological:
- Myoclonus (>50%)
- Excitatory spikes on EEG (~20%), seizures (rare).
- Awareness post-intubation due to rapid offset if ongoing analgo-sedation is not commenced promptly.
- decreases CMRO2, cerebral blood flow (20-30% on induction) and ICP.
- Decreases intraocular pressure
- Not neuroprotective in animal studies
- Endocrine:
- Transient adrenal suppression from single induction boluses, chronic adrenal insufficiency can occur from etomidate infusions and recreational abuse
- Respiratory:
- Hypoventilation, apnea.
- Case reports of laryngospasm
- Gastrointestinal:
- Nausea, vomiting (more common than propofol and thiopentone)
- Other
- Pain on injection (20-50%) and phlebitis (polyethylene glycol formulations).
PHARMACOKINETICS
- Absorption
- Rapid onset within 1 minute of IV administration.
- Distribution
- High lipid solubility, rapid brain uptake. Distribution t1/2 = 2-4 min; central VDss = 2.5-4.5 L/kg. Very large peripheral volume of distribution, 74.9 litres/kg.
- Protein binding 77% (especially albumin).
- Modest increase in context-sensitive half-time with prolonged infusion.
- Metabolism
- Hydrolyzed by hepatic and plasma esterases to inactive metabolites.
- Excretion
- Primarily renal (78%), with some biliary excretion (22%).
- Clearance 18-25 mL/kg/min. Elimination t1/2 2.9-5.3h.
PREGNANCY AND LACTATION
- Pregnancy: Use only if clearly needed; potential risk to the fetus.
- Lactation: Small amounts excreted in breast milk; generally considered safe for breastfeeding once the mother is fully awake.
DOSE ADJUSTMENTS IN ORGAN FAILURES
- Renal Impairment: No specific adjustments; monitor for prolonged effects.
- Liver Impairment: Use with caution due to hepatic metabolism.
- Renal Replacement Therapy: No specific guidelines; monitor closely.
EVIDENCE
RSI trial (Casey et al, 2025)
- Multi-center randomised trial in 8 EDs and ICUs in North America
- Pragmatic design, not blinded
- N = 2,444 non-trauma critically ill patients.
- Compared etomidate (0.2-0.3 mg/kg IV actual body weight) and ketamine (1-2 mg/kg IV actual body weight) when used for emergency intubation
- Clinicians selected the actual dose given using a weight-based nomogram
- Findings
- no mortality difference
- Cardiovascular collapse (composite of new/ increased vasopressors or SBP <65 mmHg within 2 minutes, or cardiac arrest) was more common with ketamine (22%) vs etomidate (17%) (NNH 20)
- Effect was greater in sepsis and in APACHE II >20 patients (NNH = 10)
- Comments and criticisms
- Excellent, well designed pragmatic trial with excellent internal validity
- Dosing used is a threat to external validity, as doses used are higher those used in many centers
- IBW dosing rather than actual body weight is recommended based on expert consensus and pharmacokinetic principles
- Many patients were overweight or living with obesity (median BMI was 26)
- About 25% of patients in both arms were administered doses higher than the maximum dose recommended by the trial nomograms.
- Conclusion:
- At the doses used in non-trauma critically ill patients,, there was mortality difference between etomidate and ketamine and there was less cardiovascular collapse with etomidate.
- Confirms etomidate as the most haemodynamically stable induction agent.
Other clinical trials comparing ketamine and etomidate as induction agents for emergency intubation:
- KETASED (2009)
- French RCT of n=449 ICU adults needing RSI; Etomidate vs Ketamine.
- Primary outcome: SOFA score at 3 days.
- Findings: No difference in SOFA score at 3 days (though difference in 95% CI was 0 to 1.1) or mortality. Confirmed etomidate causes adrenal suppression.
- EvK (2021/22)
- European RCT of n=801 critically ill adults needing emergency intubation; Etomidate vs Ketamine.
- Primary outcome: 30‑day survival.
- Findings: No survival benefit of ketamine (though there was a statistically significant 7-day mortality difference in favour of ketamine that did not persist to 30 days). Hemodynamic effects were similar.; Confirmed etomidate causes adrenal suppression.
Systematic reviews and meta-analysis prior to the RSI trial provide mixed conclusions
- Kotani et al (2023)
- found no clear mortality difference but confirmed etomidate’s adrenal suppression risk. Bayesian analysis suggested that a mortality benefit in favour of ketamine was likely. Confirmed the adrenal suppression effect of etomidate, in comparison with ketamine.
- Greer et al (2025)
- found that neither ketamine nor etomidate as induction agents improved survival; ketamine may worsen cardiovascular outcomes (arrhythmias and hypotension), while confirming that etomidate impairs adrenal function.
CONTROVERSIES
- Historically, there was concern about the potential for increased mortality with etomidate, especially in patients with sepsis. However, there was no signal for harm from etomidate in the 2025 RSI trial.
- Etomidate boluses commonly cause transient adrenal suppression (<24-72h), but the clinical significance is uncertain
- Etomidate is not available in a number of countries (including Australia) due to historical safety concerns of regulatory bodies.
- Etomidate can be a recreational drug of abuse. It has been used in vaping fluids (“space oil”). Recurrent use can lead to adrenal insufficiency.
PRACTICAL TIPS
- Etomidate has rapid onset and offset. Ensure prompt initiation of post-intubation analgo-sedation to minimise the risk of awareness.
- Never use etomidate infusions for ongoing post-intubation sedation due to the risk of adrenal insufficiency.
- Hemorrhagic shock produced minimal changes in the pharmacokinetics and no change in the pharmacodynamics of etomidate in swine (Johnson, 2003). These findings suggest , unlike other sedative hypnotics and opioids, minimal adjustment in the dose of etomidate is required to achieve the same drug effect during hemorrhagic shock. A proportional decrease in the dose of etomidate in haemorrhagic shock may lead to greater risk of awareness than for other agents (cf. propofol dosing should be markedly decreased by up to 80% in haemorrhagic shock).
REFERENCES
Journal articles
- Casey JD, Seitz KP, Driver BE, et al. Ketamine or Etomidate for Tracheal Intubation of Critically Ill Adults. N Engl J Med. Published online December 9, 2025. doi:10.1056/NEJMoa2511420 PMID: 41369227.
- Forman SA. Clinical and molecular pharmacology of etomidate. Anesthesiology. 2011;114(3):695-707. doi:10.1097/ALN.0b013e3181ff72b5 PMID: 21263301 PMCID: PMC3108152
- Greer A, Hewitt M, Khazaneh PT, et al. Ketamine Versus Etomidate for Rapid Sequence Intubation: A Systematic Review and Meta-Analysis of Randomized Trials. Crit Care Med. 2025;53(2):e374-e383. doi:10.1097/CCM.0000000000006515 PMID: 39570063
- Jabre P, Combes X, Lapostolle F, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374(9686):293-300. doi:10.1016/S0140-6736(09)60949-1 PMID: 19573904
- Johnson KB, Egan TD, Layman J, Kern SE, White JL, McJames SW. The influence of hemorrhagic shock on etomidate: a pharmacokinetic and pharmacodynamic analysis. Anesth Analg. 2003;96(5):1360-1368. doi:10.1213/01.ANE.0000055804.30509.69 PMID: 12707134
- Koroki T, Kotani Y, Yaguchi T, et al. Ketamine versus etomidate as an induction agent for tracheal intubation in critically ill adults: a Bayesian meta-analysis. Crit Care. 2024;28(1):48. Published 2024 Feb 17. doi:10.1186/s13054-024-04831-4 PMID: 38368326
- Matchett G, Gasanova I, Riccio CA, et al. Etomidate versus ketamine for emergency endotracheal intubation: a randomized clinical trial. Intensive Care Med. 2022;48(1):78-91. doi:10.1007/s00134-021-06577-x PMID: 34904190
FOAM and web resources
- Critical Care Reviews – RSI trial at CCR Down Under 2025 (trial presentation, editorial, and panel discussion with Q&A)
- FDA Data sheet for AMIDATE (etomidate)
- EMCrit Wee – The RSI Trial (2025)
- ICU Ed and Todd Cast – CCR Down Under: RSI with Jon Casey (2025)

Critical Care
Compendium
CCC Pharmacology Series
Respiratory: Bosentan, Delivery of B2 Agonists in Intubated Patients, Nitric Oxide, Oxygen, Prostacyclin, Sildenafil
Cardiovascular: Adenosine, Adrenaline (Epinephrine), Amiodarone, Classification of Vasoactive drugs, Clevidipine, Digoxin, Dobutamine, Dopamine, Levosimendan, Levosimendan vs Dobutamine, Milrinone, Noradrenaline, Phenylephrine, Sodium Nitroprusside (SNiP), Sotalol, Vasopressin
Neurological: Dexmedetomidine, Ketamine, Levetiracetam, Lignocaine, Lithium, Midazolam, Physostigmine, Propofol, Sodium Valproate, Sugammadex, Thiopentone
Endocrine: Desmopressin, Glucagon Therapy, Medications and Thyroid Function
Gastrointestinal: Octreotide, Omeprazole, Ranitidine, Sucralfate, Terlipressin
Genitourinary: Furosemide, Mannitol, Spironolactone
Haematological: Activated Protein C, Alteplase, Aprotinin, Aspirin, Clopidogrel, Dipyridamole, DOACs, Factor VIIa, Heparin, LMW Heparin, Protamine, Prothrombinex, Tenecteplase, Tirofiban, Tranexamic Acid (TXA), Warfarin
Antimicrobial: Antimicrobial Dosing and Kill Characteristics, Benzylpenicillin, Ceftriaxone, Ciprofloxacin, Co-trimoxazole / Bactrim, Fluconazole, Gentamicin, Imipenem, Linezolid, Meropenem, Piperacillin-Tazobactam, Rifampicin, Vancomycin
Analgesic: Alfentanil, Celecoxib, COX II Inhibitors, Ketamine, Lignocaine, Morphine, NSAIDs, Opioids, Paracetamol (Acetaminophen), Paracetamol in Critical Illness, Tramadol
Miscellaneous: Activated Charcoal, Adverse Drug Reactions, Alkali Therapies, Drug Absorption in Critical Illness, Drug Infusion Doses, Epidural Complications, Epidural vs Opioids in Rib Fractures, Magnesium, Methylene Blue, Pharmacology and Critical Illness, PK and Obesity, PK and ECMO, Sodium Bicarbonate Use, Statins in Critical Illness, Therapeutic Drug Monitoring, Weights in Pharmacology
Toxicology: Digibind, Flumazenil, Glucagon Therapy, Intralipid, N-Acetylcysteine, Naloxone, Propofol Infusion Syndrome
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC
