Obesity and Pharmacokinetics
Reviewed and revised 6 March 2015
OVERVIEW
- obesity affects all four aspects of pharmacokinetics
- As drug administration based on total body weight can result in in underdosing or overdosing, depending on the characteristics of the drug, weight-based dosing scalars must be considered
- Lean body weight is the optimal scalar for most IV opioids and anaesthetics
- The pharmacodynamic profile of drugs may also be affected, e.g. the risk of respiratory depression and loss of airway patency is greater with sedatives and narcotics
- careful therapeutic drug monitoring is important in obese patients
- morbidly obese people are often excluded from clinical trials during the drug development process so data is limited on the correct dosing of many drugs, and clinical judgement, combined with interpretation of drug pharmacokinetics, is often required
Total Body Weight (TBW) is the patient’s actual weight.
Ideal Body Weight (IBW):
- Males = height – 100
- Females = height – 110
Lean Body Weight (LBW):
- Males = 50 + 0.9kg for every cm over 150cm
- Females = 45 + 0.9kg for every cm over 150cm
ABSORPTION
- increased absorption of oral medications (increased gastric emptying)
- difficult IV access in the obese
- decreased SC absorption due to poor subcutaneous blood supply
- IM administration may fail if needles are too short
DISTRIBUTION
- markedly affected by ratio of adipose tissue to lean body mass if lipid soluble
- increased Vd of lipid soluble drugs
-> dose lipid soluble drugs on actual body weight
(remifentanil is an exception, although lipophilic Vd does not change – use IBW or LBW) - no change in Vd of H2O soluble drugs (but blood, ECF, body organ and connective tissue volume are also increased)
-> dose on ideal or lean body weight - accumulation of lipophilic drugs in fat stores
- may increase dose required to gain effect
- total body water may be increased by resuscitation volume
- Cmax reduced
- altered protein binding
METABOLISM
- variable effects
- increases in cytochrome P450 2E1 activity and phase II conjugation activity
- more likely to be affected by critical illness with drug interactions
- reduced hepatic blood flow
ELIMINATION
- T1/2 increased of lipid soluble drugs due to accumulation
- obese patients with normal renal function have increased GFRs
-> increased clearance of drugs excreted by the kidneys - co-existing disease will effect this (e.g. nephropathy associated with diabetes and hypertension)
- calculated and measured creatinine clearance correlate poorly in obesity and in critical illness
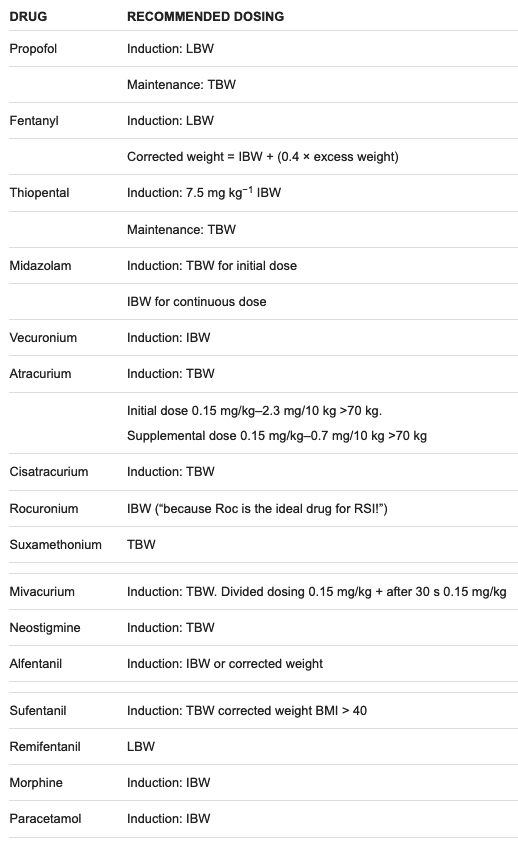
DOSING
Dosing of common anaesthetic drugs in the obese
References and Links
CCC Pharmacology Series
Respiratory: Bosentan, Delivery of B2 Agonists in Intubated Patients, Nitric Oxide, Oxygen, Prostacyclin, Sildenafil
Cardiovascular: Adenosine, Adrenaline (Epinephrine), Amiodarone, Classification of Vasoactive drugs, Clevidipine, Digoxin, Dobutamine, Dopamine, Levosimendan, Levosimendan vs Dobutamine, Milrinone, Noradrenaline, Phenylephrine, Sodium Nitroprusside (SNiP), Sotalol, Vasopressin
Neurological: Dexmedetomidine, Ketamine, Levetiracetam, Lignocaine, Lithium, Midazolam, Physostigmine, Propofol, Sodium Valproate, Sugammadex, Thiopentone
Endocrine: Desmopressin, Glucagon Therapy, Medications and Thyroid Function
Gastrointestinal: Octreotide, Omeprazole, Ranitidine, Sucralfate, Terlipressin
Genitourinary: Furosemide, Mannitol, Spironolactone
Haematological: Activated Protein C, Alteplase, Aprotinin, Aspirin, Clopidogrel, Dipyridamole, DOACs, Factor VIIa, Heparin, LMW Heparin, Protamine, Prothrombinex, Tenecteplase, Tirofiban, Tranexamic Acid (TXA), Warfarin
Antimicrobial: Antimicrobial Dosing and Kill Characteristics, Benzylpenicillin, Ceftriaxone, Ciprofloxacin, Co-trimoxazole / Bactrim, Fluconazole, Gentamicin, Imipenem, Linezolid, Meropenem, Piperacillin-Tazobactam, Rifampicin, Vancomycin
Analgesic: Alfentanil, Celecoxib, COX II Inhibitors, Ketamine, Lignocaine, Morphine, NSAIDs, Opioids, Paracetamol (Acetaminophen), Paracetamol in Critical Illness, Tramadol
Miscellaneous: Activated Charcoal, Adverse Drug Reactions, Alkali Therapies, Drug Absorption in Critical Illness, Drug Infusion Doses, Epidural Complications, Epidural vs Opioids in Rib Fractures, Magnesium, Methylene Blue, Pharmacology and Critical Illness, PK and Obesity, PK and ECMO, Sodium Bicarbonate Use, Statins in Critical Illness, Therapeutic Drug Monitoring, Weights in Pharmacology
Toxicology: Digibind, Flumazenil, Glucagon Therapy, Intralipid, N-Acetylcysteine, Naloxone, Propofol Infusion Syndrome
LITFL
- CCC – Obesity and trauma
- CCC – Obesity and Critical Illness
- CCC – Complications of Obesity
- CCC – Nutrition of the Critically Ill Obese Patient
- CCC – Bariatric patient hot case
Journal articles and textbooks
- De Baerdemaeker LEC, Mortier EP, Struys MMRF. Pharmacokinetics in obese patients Contin Educ Anaesth Crit Care Pain 2004; 4 (5): 152-155. [Free Full Text]
- Erstad BL. Dosing of medications in morbidly obese patients in the intensive care unit setting. Intensive Care Med. 2004 Jan;30(1):18-32. PMID: 14625670.
- Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004 Aug;58(2):119-33. PMC1884581.
- Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71-87. PMID: 20067334.
- Ingrande J, Lemmens HJM. Dose adjustment of anaesthetics in the morbidly obese. BJA. 2010; 105 (suppl 1): i16-i23. [Free Full Text]
- Leykin Y, Miotto L, Pellis T. Pharmacokinetic considerations in the obese. Best Pract Res Clin Anaesthesiol. 2011 Mar;25(1):27-36. PMID: 21516911.
- Medico CJ, Walsh P. Pharmacotherapy in the critically ill obese patient. Crit Care Clin. 2010 Oct;26(4):679-88. PMID: 20970057.
Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC
