Blood Gas Interpretation
The blood gas is used to rapidly assess ventilatory function and identify acid-base disorders – and will also generally provide point-of-care testing of a number of values such as electrolytes, blood glucose and haemoglobin.
Interpretation occurs in a systematic manner to identify the primary disorder, secondary disorders and therefore the differential diagnoses that could be contributing to the patient’s clinical state.
Use the following steps and flowchart to approach blood gas interpretation in a comprehensive and structured manner:

Interpreting the blood gas in a step-wise manner:
- Step 1. pH
- Step 2. Assess the pCO2 and HCO3 to identify the primary disorder
- Step 3. Consider the cause of the primary disorder
- Step 4. Assess for secondary processes
- Step 5. Apply corrections to certain measured values
Step 1. pH
A normal pH is between 7.35 and 7.45 (use 7.4 as the ‘normal’ value for all calculations)
- If pH < 7.35 there is acidaemia
- If pH > 7.45 there is alkalaemia
If pH is normal – there could be no acid-base disorders occurring, or there could be multiple processes occurring concurrently
Remember – acidaemia/alkalaemia describe the pH status of the blood, not the process(es) driving the abnormality
Step 2. Assess the pCO2 and HCO3 to identify the primary disorder
- A normal pCO2 is between 35 – 45mmHg (use 40mmHg as the ‘normal’ value for all calculations)
- A normal HCO3 is between 22 – 26mmol/L (use 24mmol/L as the ‘normal’ value for all calculations)
If there is acidaemia:
- An elevated pCO2 indicates a respiratory acidosis
- A reduced HCO3 indicates a metabolic acidosis
If there is alkalaemia
- A reduced pCO2 indicates a respiratory alkalosis
- An elevated HCO3 indicates a metabolic alkalosis
Step 3. Consider the cause of the primary disorder
Respiratory acidosis (elevated pCO2)
Respiratory acidosis (elevated pCO2)
Consider causes of hypoventilation
- CNS depression (head injury, stroke, drugs)
- Respiratory depression (myopathy, spinal cord injury, drugs)
- Hypoventilation (pain, chest wall injury/deformity, raised intra-abdominal pressures)
- Respiratory failure (pneumonia, pneumothorax, oedema, bronchial obstruction)
- Airway obstruction
- Chronic respiratory acidosis – COPD, restrictive lung disease
Metabolic acidosis (reduced HCO3)
Metabolic acidosis (reduced HCO3)
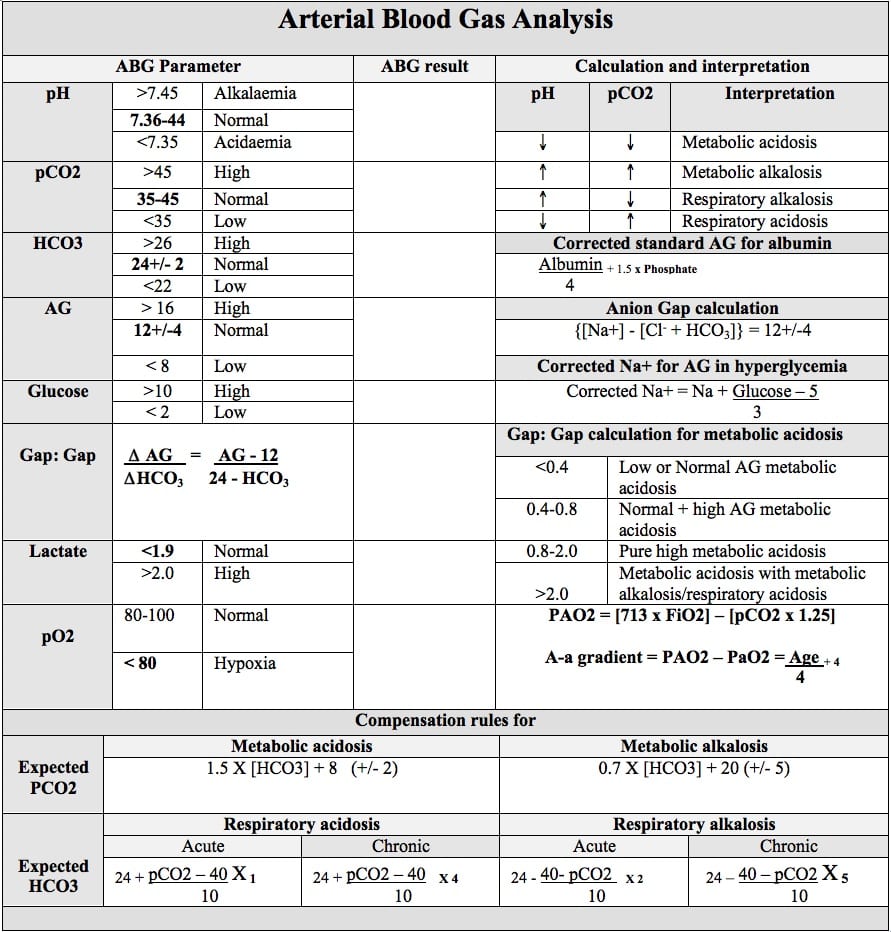
The first step involves calculating the anion gap, where there are different categories of differentials for either a normal anion gap metabolic acidosis (NAGMA) or a high anion gap metabolic acidosis (HAGMA)
Anion Gap = [Na+] – ([Cl–] + [HCO3–])
Normal anion gap metabolic acidosis (NAGMA)
A normal anion gap is 12 ± 4. Differentials for a normal anion gap metabolic acidosis can be remembered with “USED CRAP”
USED CRAP
- Ureterostomy
- Small bowel fistula
- Extra chloride (hyperchloraemic metabolic acidosis)
- Diarrhoea
- Carbonic anhydrase inhibitor
- Renal tubular acidosis
- Addison disease
- Pancreatic duodenal fistula
The anion gap can also be low (<3) or negative, with the differentials for a low or negative anion gap metabolic acidosis including:
- Analytical error (severe hypernatraemia, pseudohyponatraemia, hyperviscosity, hyperlipidaemia)
- Reduced unmeasured anions (dilution, hypoalbuminaemia)
- Increased unmeasured cations (lithium, calcium, magnesium, potassium)
- Pseudohyperchloraemia (bromide, iodide, salicylates, thiocyanate)
High anion gap metabolic acidosis (HAGMA)
The differentials for a high anion gap (>16) metabolic acidosis can be remembered with “CAT MUD PILES” or more simply “Left Total Knee Replacement (L TKR)”
CAT MUD PILES
- Carbon monoxide, cyanide
- Alcoholic ketoacidosis
- Toluene
- Methanol, metformin (phenformin)
- Uraemia
- Diabetic ketoacidosis, D-lactic acidosis
- Paracetamol, pyroglutamic acid, paraldehyde, propylene glycol
- Isoniazid, iron
- Lactate
- Ethanol, ethylene glycol
- Salicylates
L TKR
- Lactate
- Toxins
- Ketones
- Renal
Respiratory alkalosis (reduced pCO2)
Respiratory alkalosis (reduced pCO2)
Related to processes that cause hyperventilation – differentials can be remembered with “CHAMPS“
- CNS disease (stroke, haemorrhage, psychogenic)
- Hypoxia (pneumonia, PE, asthma, altitude)
- Anxiety, pain
- Mechanical or excessive ventilation
- Progesterone, pregnancy
- Salicylates and sepsis
Metabolic alkalosis (elevated HCO3)
Metabolic alkalosis (elevated HCO3)
Differentials can be remembered with “CLEVER PD“
- Contraction (volume contraction)
- Liquorice, laxative abuse
- Endocrine (Conn syndrome, Cushing syndrome)
- Vomiting, GI losses, chloride-wasting enteropathy, CF
- Excess alkali (antacids)
- Renal (Bartter syndrome)
- Post-hypercapnia
- Diuretics
“Chloride responsive”: Chloride losing conditions (GI losses, diuretic therapy, cystic fibrosis and chloride-wasting enteropathy)
“Chloride unresponsive”: Hyperaldosteronism, Cushing’s syndrome, exogenous mineralocorticoids (licorice, fludrocortisone), renal artery stenosis, renin-secreting tumours
Step 4. Assess for secondary processes
If there is a primary metabolic disorder, there is an expected respiratory compensation that can be calculated, and vice versa for a primary respiratory disorder. Respiratory compensation occurs quickly (within 30 minutes and complete within 12-24 hours), whereas metabolic compensation begins within 5-10 minutes (with whole body buffering) but requires 3-5 days to complete with further renal processes.
If the degree of compensation is not appropriate (e.g. the expected pCO2 or HCO3 is above or below the measured value) this is indicative of concurrent additional acid base disorders occurring.
A respiratory acidosis is compensated for by increasing HCO3
- Calculate the expected HCO3 using 1-2-3-4-5 rule
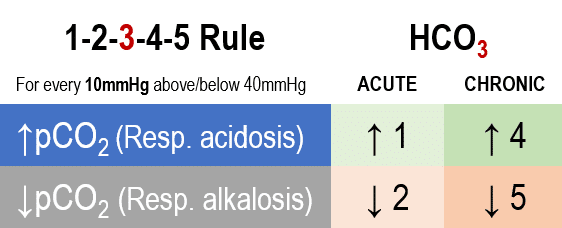
- This rule states that for acute respiratory acidosis the expected HCO3 will increase by 1mmol/L (from the normal value of 24) for every 10mmHg that the pCO2 rises above normal (40mmHg)
- For chronic respiratory acidosis the expected HCO3 will increase by 4mmol/L for every 10mmHg rise in pCO2 above normal
A metabolic acidosis is compensated for by reducing pCO2
- Calculate the expected pCO2 using Winter’s formula:
pCO2 expected = (1.5 x [HCO3–]) + 8 ±2
- The expected pCO2 can also be approximated using the two digits to the right of the decimal point (e.g. expected pCO2 = 25 if the pH = 7.25)
- If the measured pCO2 is more than the expected pCO2 – there is also a respiratory acidosis
Delta ratio
In the context of a metabolic acidosis, a further calculation can be performed to assess whether the metabolic acidosis is due to single process or a mixed acid-base disorder. This is called the delta ratio, and reflects whether the change in the anion gap from normal (delta AG) is proportional to the expected change in the HCO3 from normal (delta HCO3).
Delta ratio = (AG – 12) / (24 – HCO3–)
If the denominator is larger (i.e. the HCO3 has changed more than the change in anion gap) this could reflect that a NAGMA is present (either by itself or concurrently with a HAGMA). If the numerator is larger (i.e. the anion gap has changed more than the change in HCO3) this could reflect that there is predominantly a HAGMA occurring – or if the ratio is very high (>2) this could reflect that there is an additional process present (such as a concurrent metabolic alkalosis or a chronic respiratory acidosis which is raising the HCO3 and reducing the delta HCO3)
Delta ratio interpretation:
| <0.4 | Pure NAGMA |
| 0.4 – 0.8 | Mixed NAGMA and HAGMA |
| 0.8 – 2.0 | Pure HAGMA |
| >2.0 | HAGMA and either metabolic alkalosis or chronic respiratory acidosis |
A respiratory alkalosis is compensated for by reducing HCO3
- Calculate the expected HCO3 using 1-2-3-4-5 rule
- This rule states that for acute respiratory alkalosis the expected HCO3 will reduce by 2mmol/L (from the normal value of 24) for every 10mmHg that the pCO2 drops below normal (40mmHg)
- For chronic respiratory alkalosis the expected HCO3 will reduce by 5mmol/L for every 10mmHg reduction in pCO2 below normal
- If the measured HCO3 is less than the expected HCO3 – there is also a metabolic acidosis occurring.
- If the measured HCO3 is greater than expected – there is also a metabolic alkalosis.
A metabolic alkalosis is compensated for by increasing pCO2
- Calculate the expected pCO2 using:
pCO2 expected = (0.7 x [HCO3–]) + 20 ± 5
- If the measured pCO2 is more than the expected pCO2 – there is also a respiratory acidosis occurring
- If the measured pCO2 is less than expected – there is a concurrent respiratory alkalosis.
Step 5. Apply corrections to certain measured values
In certain acid base disturbances, particular measured values may be falsely normal/abnormal and correction factors can be applied to assess for further abnormalities.
Albumin correction of anion gap
Albumin is an anion, therefore hypoalbuminaemia may under-estimate the anion gap. To correct the anion gap for hypoalbuminaemia – for every 10g/L that the albumin is below normal (40g/L) add 2.5 to the calculated anion gap
Anion gapAlbumin corrected = Anion gap + 0.25 (40 – Albumin)
Osmolar gap
The osmolar gap technically compares two different values – the osmolality (measured in the laboratory with freezing point depression, mOsm/kg), and the osmolarity (calculated, mOsm/L). Normally the gap between these two values is less than 10, and if the osmolar gap is elevated it can reflect the presence of an abnormal solute (e.g. toxic alcohol)
Osmolarity = (2x [Na+]) + urea + glucose + (1.25 x EtOH)
Note: Ethanol must be converted to SI units (mmol/L)
EtOH (mmol/L) = EtOH (%) x 217 = EtOH (mg/dL) ÷ 4.6
Exogenous agents associated with an elevated osmolar gap
- Acetone
- Ethanol
- Ethylene glycol
- Glycerol
- Glycine
- Isopropyl alcohol
- Maltose
- Mannitol
- Methanol
- Propylene glycol
Other conditions (non-toxicological) associated with an elevated osmolar gap
- Alcoholic ketoacidosis
- Chronic renal failure
- Diabetic ketoacidosis
- Hyperlipidaemia
- Hyperproteinaemia
- Severe lactic acidosis
- Shock
- Trauma and burns
Sodium correction in hyperglycaemia
In patients with marked hyperglycaemia, the elevated serum glucose raises the serum tonicity (as the glucose cannot enter cells) which pulls water out of cells and expands the extracellular water compartment – thereby lowering the concentration of sodium.
The corrected serum sodium can be calculated, which represents what the serum sodium concentration would be if the glucose level was reduced back to normal. If the corrected sodium is in a normal range – the patient does not have a concurrent hypotonic hyponatraemia.
[Na] corrected = [Na] + 1.5x([Glucose – 5.5]/5.5)
Roughly approximate to [Na] corrected = [Na] + (Glucose – 5)/3
Potassium correction with pH abnormalities
Acid-base disturbances will affect the serum concentration of potassium – where acidaemia will increase the measured serum potassium by causing extracellular shift, and alkalaemia will reduce the measured serum potassium by causing intravascular shift.
The described relationship is that for every 0.1 unit change in pH, there will be a 0.6mEq/L change in serum potassium
K+corrected = [K+] measured – 0.6 ([7.4 – pH] / 0.1 )
References and Links
Introduction to ICU Series
Introduction to ICU Series Landing Page
DAY TO DAY ICU: FASTHUG, ICU Ward Round, Clinical Examination, Communication in a Crisis, Documenting the ward round in ICU, Human Factors
AIRWAY: Bag Valve Mask Ventilation, Oropharyngeal Airway, Nasopharyngeal Airway, Endotracheal Tube (ETT), Tracheostomy Tubes
BREATHING: Positive End Expiratory Pressure (PEEP), High Flow Nasal Prongs (HFNP), Intubation and Mechanical Ventilation, Mechanical Ventilation Overview, Non-invasive Ventilation (NIV)
CIRCULATION: Arrhythmias, Atrial Fibrillation, ICU after Cardiac Surgery, Pacing Modes, ECMO, Shock
CNS: Brain Death, Delirium in the ICU, Examination of the Unconscious Patient, External-ventricular Drain (EVD), Sedation in the ICU
GASTROINTESTINAL: Enteral Nutrition vs Parenteral Nutrition, Intolerance to EN, Prokinetics, Stress Ulcer Prophylaxis (SUP), Ileus
GENITOURINARY: Acute Kidney Injury (AKI), CRRT Indications
HAEMATOLOGICAL: Anaemia, Blood Products, Massive Transfusion Protocol (MTP)
INFECTIOUS DISEASE: Antimicrobial Stewardship, Antimicrobial Quick Reference, Central Line Associated Bacterial Infection (CLABSI), Handwashing in ICU, Neutropenic Sepsis, Nosocomial Infections, Sepsis Overview
SPECIAL GROUPS IN ICU: Early Management of the Critically Ill Child, Paediatric Formulas, Paediatric Vital Signs, Pregnancy and ICU, Obesity, Elderly
FLUIDS AND ELECTROLYTES: Albumin vs 0.9% Saline, Assessing Fluid Status, Electrolyte Abnormalities, Hypertonic Saline
PHARMACOLOGY: Drug Infusion Doses, Summary of Vasopressors, Prokinetics, Steroid Conversion, GI Drug Absorption in Critical Illness
PROCEDURES: Arterial line, CVC, Intercostal Catheter (ICC), Intraosseous Needle, Underwater seal drain, Naso- and Orogastric Tubes (NGT/OGT), Rapid Infusion Catheter (RIC)
INVESTIGATIONS: ABG Interpretation, Echo in ICU, CXR in ICU, Routine daily CXR, FBC, TEG/ROTEM, US in Critical Care
ICU MONITORING: NIBP vs Arterial line, Arterial Line Pressure Transduction, Cardiac Output, Central Venous Pressure (CVP), CO2 / Capnography, Pulmonary Artery Catheter (PAC / Swan-Ganz), Pulse Oximeter

Critical Care
Compendium
Physician in training. German translator and lover of medical history.

Thank you… very helpful
The aniongap is usually calculated without potassium en that value is used to calculate the delta ratio. What if the potassium is high? Is that a reason to include it, which will give a change in the delta-ratio compared to not using the potassium?
This question was raised when interpreting the following ABG:
pH 6.98; pCO2 14 mmHg (1.9 kPa); HCO3 3 mmol/l; Na 138 mmol/l; K 7.0 mmol/l; Cl 114 mmol/l
With kind regards,
René