Arterial line and Pressure Transducer
OVERVIEW
- arterial catheter connected to a pressure transducer
USES
- blood pressure (systolic, diastolic, mean and pulse pressure)
- arterial blood sampling
Specific indications
- Labile blood pressure
- Anticipation of haemodynamic instability
- Titration of vasoactive drugs
- Frequent blood sampling
- Morbid obesity (unable to fit an appropriately sized NIBP cuff)
DESCRIPTION
- arterial line
- 48 inches of non-compressible rigid-walled, fluid filled tubing
- pressure transducer and automatic flushing system
- pressure bag and automated slow infusion (1-3mL/h) of pressurised saline
- electronic transducer amplifier display
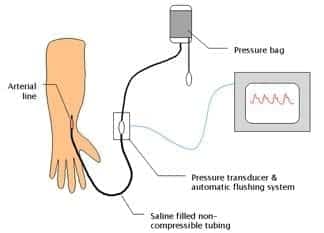
METHOD OF INSERTION AND/OR USE
Mechanism
- fluctuations of vascular pressure cause a pulsation of the saline column
- displaces electromanometer’s diaphragm which has a built in strain gauge (Wheatstone bridge principle)
- deformation leads to a change in resistance of the strain gauge which is sensed electronically
- wave form built up by Fourier analysis from sinusoids or simple wave forms
- wave forms differ depending on where the cannula is inserted
Calibrating (‘zeroing’)
- ensure the transducer pressure tubing and flush solution are correctly assembled and free of air bubbles
- place transducer at level of the right atrium
- ‘off to patient, open to air (atmosphere)’
- press ‘zero’ -> sets atmospheric pressure as zero reference point
- whenever patient position is altered the transducer height should be altered
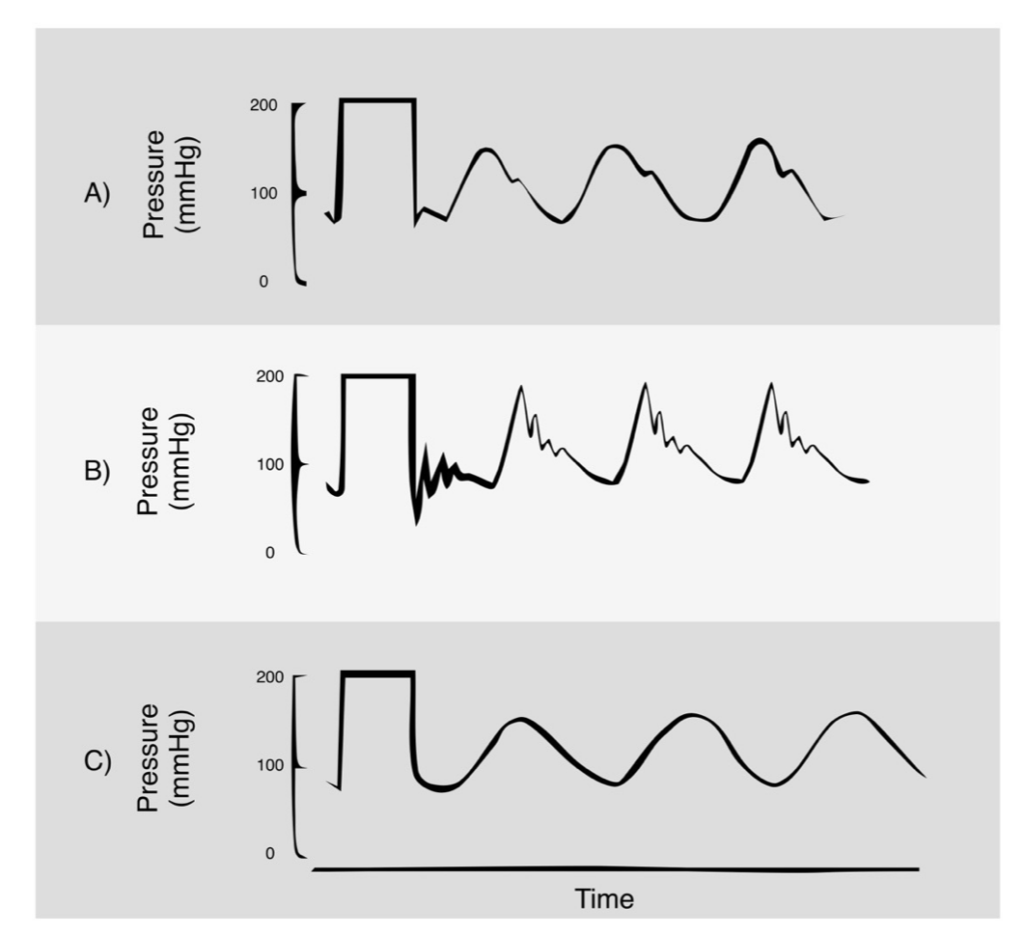
Square wave test
- aka fast flush test
- snap flush to generate square wave
- check for oscillations as an indicator of the harmonic characteristics of the system
- usually only 1 oscillation before returning to baseline
- 2 or more oscillations before returning to baseline (underdamped)
- if no oscillations (overdamped – response speed is too slow)
ACCURACY AND MEASUREMENT ERRORS
Conditions that must be met to ensure accuracy
- cannula properly placed within the lumen of an unobstructed artery (ie. no spasm, thrombus, atheroma proximal to cannula)
- cannula not kinked or obstructed
- cannula connected by short, rigid, wide-bore tubing to the transducer
- no air bubbles in tubing or transducer
- interface from fluid to transducer accurately transmits deflections
- transducer has adequate frequency response (natural frequency > 100Hz)
- transducer is leveled and zeroed to desired point (ie. left atrium)
- no zero drift
- monitor calibrated accurately
Common sources of error
- bubbles in catheter-transducer system -> decreased resonant frequency
- clotting in arterial catheter
- elastic walls causes increased damping
- cannula won’t flush – kinked, clotted, tissued
OTHER INFORMATION
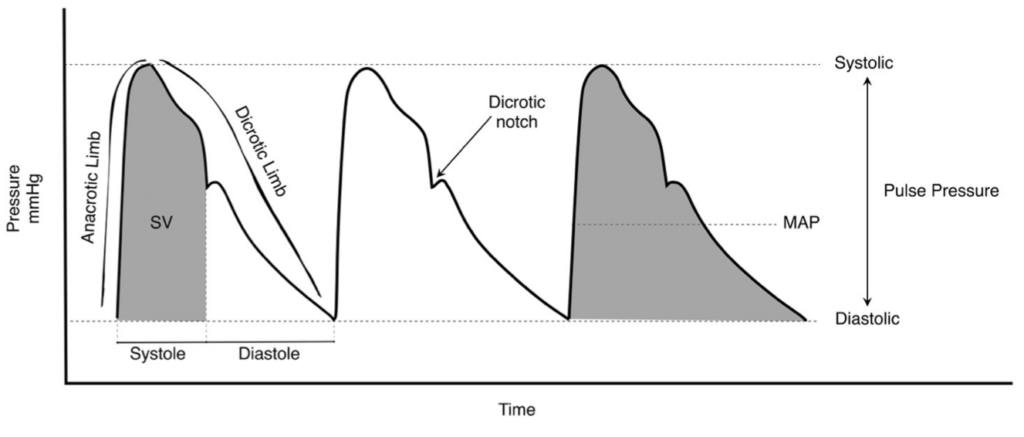
Information other than blood pressure can be obtained:
- pulse rate and rhythm
- effects of dysrhythmia on perfusion
- ECG lead disconnection
- continuous cardiac output using pulse contour analysis
- specific wave form morphologies might be diagnostic
— e.g. slow rising = AS, pulsus alternans = tamponade - pulse pressure variation (suggests fluid responsiveness)
- steeper upstroke of pulse pressure = increased contractility
- area under upstroke = SV
- steep downstroke = low SVR
Advantages of using MAP rather than SBP/DBP
- least dependent on measurement site or technique (whether invasive or not)
- least altered by damping
- determines tissue blood flow via autoregulation
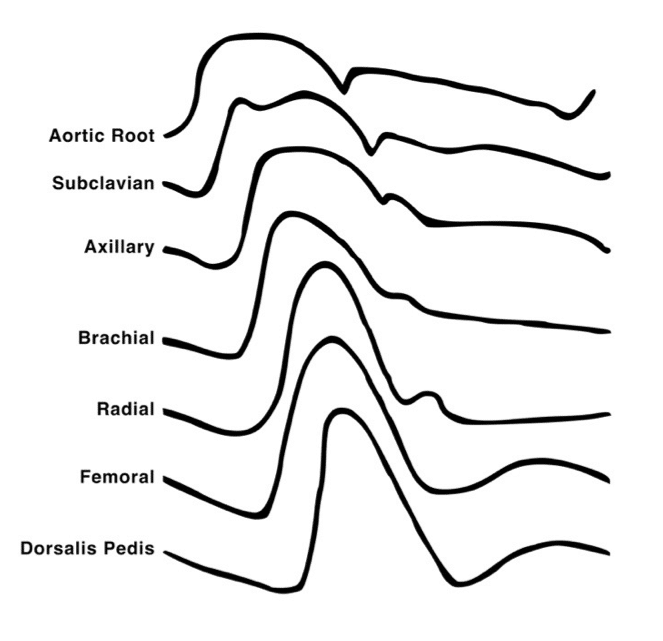
Variation in arterial waveform at different sites
- arterial waveform morphology varies with site of measurement as a result of the physical characteristics of the vascular tree (impedance and harmonic resonance)
- The following changes occur as the arterial pressure wave travels peripherally from the central aorta to the periphery:
- arterial upstroke becomes steeper
- systolic peak becomes higher (“distal pulse amplification”)
- dicrotic notch appears later
- diastolic wave becomes more prominent
- end-diastolic pressure becomes lower
- pulse pressure becomes wider
- however the MAP in the aorta remains slightly greater in the aorta than at peripheral sites (as expected for continuous blood flow from central to peripheral vessels)
- the arrival of the pulse is delayed at peripheral sites compared to the central aorta
- e.g. systolic pressure upstroke begins ~ 60 msec later in the radial artery than the aorta
COMPLICATIONS
- Pain
- thrombosis and distal ischaemia
- infection
- increased diagnostic blood loss and anemia
- retrograde air embolism
- inadvertent drug/air injection
- haematoma (+/- nerve compression)
- retroperitoneal haematoma (femoral)
- bowel perforation (femoral)
- vessel damage may lead to stricture and prevent future AV fistula formation for haemodialysis
- pseudo-aneurysm
- arterial dissection
- arteriovenous fistula
EVIDENCE
- A 2014 observational study using propensity matching based on the Project IMPACT database found no mortality benefit for use of arterial catheters in medical ICU patients requiring mechanical ventilation.
VIDEO
Example of pressure transducer set-up demonstrated by Scott Weingart:
References and Links
Introduction to ICU Series
Introduction to ICU Series Landing Page
DAY TO DAY ICU: FASTHUG, ICU Ward Round, Clinical Examination, Communication in a Crisis, Documenting the ward round in ICU, Human Factors
AIRWAY: Bag Valve Mask Ventilation, Oropharyngeal Airway, Nasopharyngeal Airway, Endotracheal Tube (ETT), Tracheostomy Tubes
BREATHING: Positive End Expiratory Pressure (PEEP), High Flow Nasal Prongs (HFNP), Intubation and Mechanical Ventilation, Mechanical Ventilation Overview, Non-invasive Ventilation (NIV)
CIRCULATION: Arrhythmias, Atrial Fibrillation, ICU after Cardiac Surgery, Pacing Modes, ECMO, Shock
CNS: Brain Death, Delirium in the ICU, Examination of the Unconscious Patient, External-ventricular Drain (EVD), Sedation in the ICU
GASTROINTESTINAL: Enteral Nutrition vs Parenteral Nutrition, Intolerance to EN, Prokinetics, Stress Ulcer Prophylaxis (SUP), Ileus
GENITOURINARY: Acute Kidney Injury (AKI), CRRT Indications
HAEMATOLOGICAL: Anaemia, Blood Products, Massive Transfusion Protocol (MTP)
INFECTIOUS DISEASE: Antimicrobial Stewardship, Antimicrobial Quick Reference, Central Line Associated Bacterial Infection (CLABSI), Handwashing in ICU, Neutropenic Sepsis, Nosocomial Infections, Sepsis Overview
SPECIAL GROUPS IN ICU: Early Management of the Critically Ill Child, Paediatric Formulas, Paediatric Vital Signs, Pregnancy and ICU, Obesity, Elderly
FLUIDS AND ELECTROLYTES: Albumin vs 0.9% Saline, Assessing Fluid Status, Electrolyte Abnormalities, Hypertonic Saline
PHARMACOLOGY: Drug Infusion Doses, Summary of Vasopressors, Prokinetics, Steroid Conversion, GI Drug Absorption in Critical Illness
PROCEDURES: Arterial line, CVC, Intercostal Catheter (ICC), Intraosseous Needle, Underwater seal drain, Naso- and Orogastric Tubes (NGT/OGT), Rapid Infusion Catheter (RIC)
INVESTIGATIONS: ABG Interpretation, Echo in ICU, CXR in ICU, Routine daily CXR, FBC, TEG/ROTEM, US in Critical Care
ICU MONITORING: NIBP vs Arterial line, Arterial Line Pressure Transduction, Cardiac Output, Central Venous Pressure (CVP), CO2 / Capnography, Pulmonary Artery Catheter (PAC / Swan-Ganz), Pulse Oximeter
Journal articles
- Gardner RM. Direct blood pressure measurement – dynamic response requirements. Anesthesiology. 1981 Mar;54(3):227-36. PMID: 7469106.
- Gershengorn HB, Wunsch H, Scales DC, Zarychanski R, Rubenfeld G, Garland A. Association Between Arterial Catheter Use and Hospital Mortality in Intensive Care Units. JAMA Intern Med. 2014 Sep 8. PMID: 25201069.
- McGhee BH, Bridges EJ. Monitoring arterial blood pressure: what you may not know. Crit Care Nurse. 2002 Apr;22(2):60-4, 66-70, 73 passim. PMID: 11961944.
- Scheer B, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002 Jun;6(3):199-204. PMC137445.
- Ward M, Langton JA. Blood pressure management. Contin Educ Anaesth Crit Care Pain (2007) 7 (4): 122-126. [Free Full Text]
FOAM and web resources
- Miers J, Gaetani D. Arterial line insertion (radial). Emergency Procedures App
- DerangedPhysiology.com — The Arterial Line
- ATOTW — Physical principles of intra-arterial blood pressure monitoring (2009) (pdf)

Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC
