CVP Measurement
Reviewed and revised 14 March 2023
OVERVIEW
Central venous pressure (CVP) is the pressure recorded from the right atrium or superior vena cava and is representative of the filling pressure of the right side of the heart
- CVP monitoring in the critically ill is established practice but the traditional belief that CVP reflects ventricular preload and predicts fluid responsiveness has been challenged by a large body of evidence
- CVP represents the driving force for filling the right atrium and ventricle
- normal is 0-6mmHg in a spontaneously breathing non-ventilated patient
MEASUREMENT
- recorded at the end of expiration
- measured by transducing the waveform of a central venous line
- electronic transducer placed & zeroed at the level of the RA (the “phlebostatic axis” – usually the 4th intercostal space in the mid-axillary line is used)
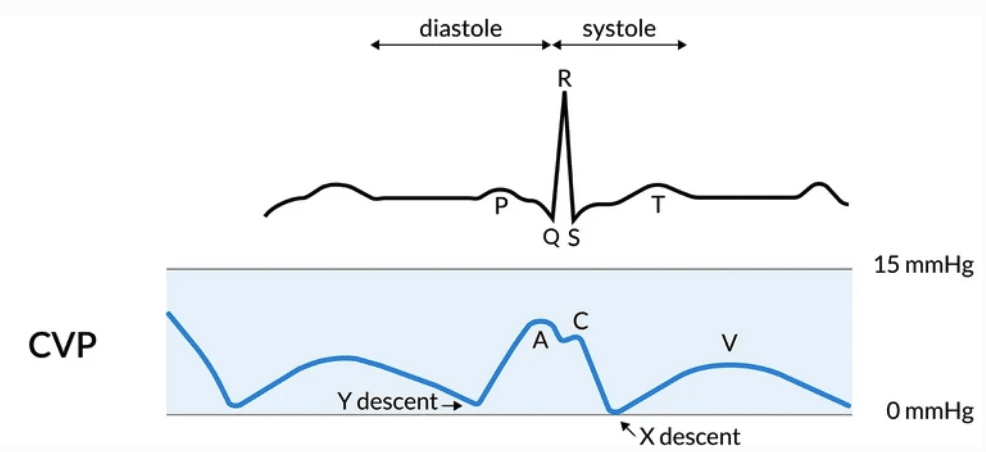
CVP WAVEFORM
a = atrial contraction
c = closing and bulging of the tricuspid valve
x = atrial relaxation, with downward movement of the tricuspid valve during ventricular contraction
v = passive filling of atrium (tricuspid valve still closed)
y = ventricular filling with opening of the tricuspid valve
DETERMINANTS OF CVP
CVP approx = RAP
VR = MSFP – RAP / Resistance to venous return
ΔCVP = ΔV / Cv
- ΔCVP-> change in CVP
- ΔV -> change in venous blood volume
- Cv -> venous compliance
Determinants include:
- right atrial pressure
- intravascular fluid volume
- venous capacitance/ tone
- mean systemic filling pressure
- right and left ventricular function and compliance
- pulmonary vascular resistance
- intrathoracic/pleural pressure
- intrabdominal pressure
USE
Value and waveform assists with diagnosis of:
- right ventricular infarction
- right heart failure and cor pulmonale
- tamponade
- Tricuspid regurgitation or stenosis
- Complete heart block
- Constrictive pericarditis
Determining:
- mechanical atrial capture with AV pacing
- presence of P waves in cases of SVT
- differential diagnosis of shock state
- correct central line placement
Do not use CVP in isolation to assess fluid responsiveness
- very poor relationship between CVP and blood volume and CVP/DeltaCVP is a poor predictor of the hemodynamic response to a fluid challenge
- interpretation of CVP should be in association with information relating to other haemodynamic variables
CAUSES OF RAISED CVP
- Right ventricular failure
- Tricuspid stenosis or regurgitation
- Pericardial effusion or constrictive pericarditis
- Superior vena caval obstruction
- Fluid overload
- Hyperdynamic circulation
- High PEEP settings
CVP WAVEFORM ANALYSIS
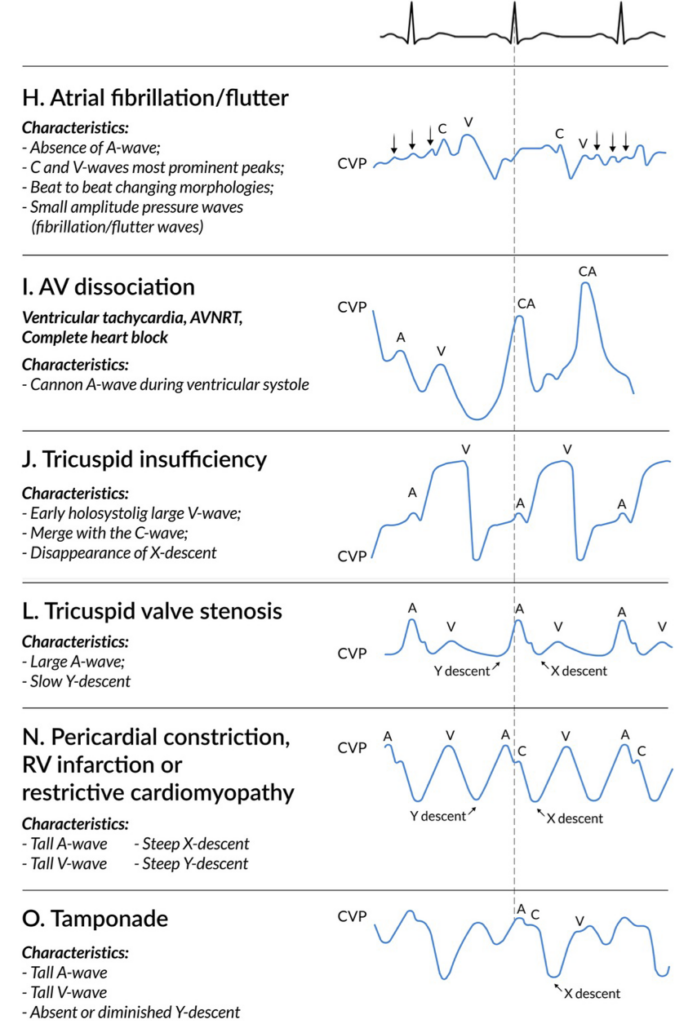
Waveform abnormalities may indicate specific pathologies:
- Dominant a wave – pulmonary hypertension, tricuspid stenosis, pulmonary stenosis
- Cannon a wave – complete heart block, ventricular tachycardia with atrio-ventricular dissociation
- Dominant v wave – tricuspid regurgitation
- Absent x descent – atrial fibrillation
- Exaggerated x descent – pericardial tamponade, constrictive pericarditis
- Sharp y descent – severe tricuspid regurgitation, constrictive pericarditis
- Slow y descent – tricuspid stenosis, atrial myxoma
- Prominent x and y descent – right ventricular infarction
FACTORS DETERMINING ACCURACY
CVP measurement should be performed at end-expiration, without fluids running
- Placement of device tip (RA, RV, SVC, SCV, femoral vein)
- Leveling – the position on the patient that you want to be zero (usually level of RA, the “phlebostatic axis”))
- Zeroing – zero means atmospheric pressure
- Calibration – comparing zero and a level above to a gold standard (mercury sphygmomanometer)
- Damping – assess by a fast flush test, preferred co-efficient around 0.7
- Frequency response of the system (intrinsic + additional tubing) -> may significantly alter damping (preferred shorter stiffer tubing)
- Running averages rather than single spontaneous readings
- PEEP — PEEP of 10 cmH20 usually results in increase of CVP by ~3 cmH20 (depends on lung compliance)
OTHER INFORMATION
- minimal difference between SVC and femoral CVP measurements in the supine patient (in the absence of intra-abdominal hypertension)
- CVP cannot be measured if fluid is running through the same lumen, but if flow rates are slow CVP is minimally affected by infusions through other lumens of the same CVC
References and Links
Introduction to ICU Series
Introduction to ICU Series Landing Page
DAY TO DAY ICU: FASTHUG, ICU Ward Round, Clinical Examination, Communication in a Crisis, Documenting the ward round in ICU, Human Factors
AIRWAY: Bag Valve Mask Ventilation, Oropharyngeal Airway, Nasopharyngeal Airway, Endotracheal Tube (ETT), Tracheostomy Tubes
BREATHING: Positive End Expiratory Pressure (PEEP), High Flow Nasal Prongs (HFNP), Intubation and Mechanical Ventilation, Mechanical Ventilation Overview, Non-invasive Ventilation (NIV)
CIRCULATION: Arrhythmias, Atrial Fibrillation, ICU after Cardiac Surgery, Pacing Modes, ECMO, Shock
CNS: Brain Death, Delirium in the ICU, Examination of the Unconscious Patient, External-ventricular Drain (EVD), Sedation in the ICU
GASTROINTESTINAL: Enteral Nutrition vs Parenteral Nutrition, Intolerance to EN, Prokinetics, Stress Ulcer Prophylaxis (SUP), Ileus
GENITOURINARY: Acute Kidney Injury (AKI), CRRT Indications
HAEMATOLOGICAL: Anaemia, Blood Products, Massive Transfusion Protocol (MTP)
INFECTIOUS DISEASE: Antimicrobial Stewardship, Antimicrobial Quick Reference, Central Line Associated Bacterial Infection (CLABSI), Handwashing in ICU, Neutropenic Sepsis, Nosocomial Infections, Sepsis Overview
SPECIAL GROUPS IN ICU: Early Management of the Critically Ill Child, Paediatric Formulas, Paediatric Vital Signs, Pregnancy and ICU, Obesity, Elderly
FLUIDS AND ELECTROLYTES: Albumin vs 0.9% Saline, Assessing Fluid Status, Electrolyte Abnormalities, Hypertonic Saline
PHARMACOLOGY: Drug Infusion Doses, Summary of Vasopressors, Prokinetics, Steroid Conversion, GI Drug Absorption in Critical Illness
PROCEDURES: Arterial line, CVC, Intercostal Catheter (ICC), Intraosseous Needle, Underwater seal drain, Naso- and Orogastric Tubes (NGT/OGT), Rapid Infusion Catheter (RIC)
INVESTIGATIONS: ABG Interpretation, Echo in ICU, CXR in ICU, Routine daily CXR, FBC, TEG/ROTEM, US in Critical Care
ICU MONITORING: NIBP vs Arterial line, Arterial Line Pressure Transduction, Cardiac Output, Central Venous Pressure (CVP), CO2 / Capnography, Pulmonary Artery Catheter (PAC / Swan-Ganz), Pulse Oximeter
Journal articles
- Bootsma, I.T., Boerma, E.C., de Lange, F. et al. The contemporary pulmonary artery catheter. Part 1: placement and waveform analysis. J Clin Monit Comput 36, 5–15 (2022). https://doi.org/10.1007/s10877-021-00662-8 [article]
- Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008 Jul;134(1):172-8. doi: 10.1378/chest.07-2331. PMID: 18628220.
- Patrick SP, Tijunelis MA, Johnson S, Herbert ME. Supraclavicular subclavian vein catheterization: the forgotten central line. West J Emerg Med. 2009 May;10(2):110-4. PMC2691520.
FOAM and web resources
- DerangedPhysiology.com — The CVC

Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC
