Pulmonary Artery Catheter
OVERVIEW
- aka Swan-Ganz catheter (or ‘the yellow snake’)
USES
- continuous cardiac output monitoring
- central temperature monitoring
- measurement of pulmonary artery pressure (can also measure RA and RV pressures during insertion)
- measurement of mixed venous saturations
- estimation of diastolic filling of left heart (normal PCWP 2-12mmHg)
Settings it is commonly used are:
- right ventricular failure
- pulmonary hypertension
- weaning failure of cardiac origin
- post-cardiac surgery
DESCRIPTION
- available in a number of sizes for both adults and children
- range from 60-110cm in length and 4-8F in calibre
- Balloon inflation volumes range from 0.5-1.5ml
- catheter is made from polyvinyl chloride that is pliable at room temperature and softens at body temperature
- catheter is marked at 1cm or 10mm increments to aid insertion
- catheters vary depending on the manufacturer but all are basically a quadruple-lumen thermodilution catheter with various additions
Components (varies with PAC type):
- Syringe (plunger withdraws only to 1.5ml)
- introducer with side port (acts as a rapid infuser)
- Sliding locking device
- Markings designating distance from tip (each broad mark represents 10cm)
- Connectors to monitor
- 8 French PVC yellow catheter
- Lumens
- blue = right atrial lumen = proximal injectate port -> terminates 30cm from the tip of the catheter lies within the right atrium when the tip of the catheter is in the pulmonary artery. This port can monitor RA pressures (RAP/ CVP) and receive the injectate for cardiac output studies. Can also be used to give fluids and drugs.
- thermistor = red/white connector -> includes a temperature-sensitive wire that terminates 4 cm proximal to tip of the catheter. The terminal portion of the wire, termed the thermistor bead, lies in one of the main pulmonary arteries when the catheter tip is properly positioned. Connection of the thermistor port to a CO monitor allows determination of a CO using thermodilution.
- white = proximal infusion port -> lumen terminates 31cm from the tip of the catheter in the right atrium and is used for infusing fluids and drugs.
- yellow = pulmonary artery lumen = distal port -> allows measurement of PA pressures and measurement of mixed venous SO2 . Caustic or hyperosmotic solutions must not be infused through this lumen.
- red = balloon inflation/deflation
- Temperature sensitive wire with thermistor bead
- Balloon surrounding tip-containing lumen in end
Other additions include:
- Catheters that offer pacing capabilities
- fiberoptics allowing continuous in vivo monitoring of mixed venous oxygen saturation
- Thermodilution catheters for continuous cardiac output measurements -> built in thermal filament that heats the surrounding blood in the right ventricle (RV) and the temperature change is detected by a thermistor located in the tip of the catheter in the pulmonary artery. This change is cross correlated with the RV thermal input to produce a thermodilution wash-out curve and the area underneath is used to get the CO. This information is used to generate graphs of CO change over time.
METHOD OF INSERTION
Technique
- sterile technique
- percutaneous Seldinger technique like CVL insertion
- preferred site: RIJ > LSCV > RSCV > LIJ (can also insert femorally)
- insert sheath (aka Cordis) first
- transducer is attached to the distal lumen to observe distinct waveform
- once the right ventricular waveform is seen the balloon is inflated to allow the PAC to progress through the right heart
- distinct waveforms plus known distance inserted aid certainty about position
- If these transitions do not occur at the estimated length then the catheter should be withdrawn and reinserted (see distances below)
- Once the pulmonary artery is reached the wedge waveform can be used to confirm position
- PAC is secured with the balloon deflated
VIDEO: Swan-Ganz insertion
Distances
- distance to right atrium: 10-15cm from the subclavian vein, 15-20cm from the jugular vein, 30-40cm from the femoral vein
- The right ventricle is another 10cm, and the pulmonary artery is another 10 cm after that, and the wedge position a further 10 cm
Pressures
- SVC/RA = 0-6 mmHg (at this point inflate balloon with 1.5 mL of air)
- RV = 25/0 mmHg
- PA = 15-30/5-15 mmHg
- PAOP = 2-10 mmHg at 40-50 cm distance (if SCV/ IJ insertion)
- look for pressure waves to change as shown below:
VIDEO: Swan-Ganz Physiology
Confirm position
- Chest x-ray – the tip should curve, without loops or knots, into a main pulmonary artery but not be more peripheral than the junction between the medial and middle third of the ipsilateral lung field in West zone 3 (below the level of the left atrium)
Balloon Inflation
- Overinflation can cause damage to the pulmonary artery and/or the balloon itself
- balloon should be inflated with a volume of 1.5 mL of air or less.
- the balloon should not remain inflated for more than four respiratory cycles when dampening/ wedging occurs
- balloon deflation should be passive – do not pull back on the balloon plunger to deflate
- Ensure that the PA waveform reappears on the monitor following balloon deflation
- When the balloon is deflated, remove the syringe and expel the air before reattaching it to the balloon inflation port – together with ensuring that the gate valve is closed, this will help prevent subsequent accidental wedging of the PA catheter
Obtaining pressure measurements
- The stopcock nearest the transducer is known as the air-fluid interface and must be positioned level with the patient’s left atrium (the phlebostatic axis). For every centimeter above the left atrium the PAC is reference the measured pressure is underestimated by 0.736 mmHg (and vice versa). In the supine patient the phlebostatic axis is identified by the intersection of 2 lines: (1) vertically from the point where the 4th intercostal space abuts the sternum (2) horizontally at the midanteriorposterior line (half the distance between the anterior and posterior aspect of the chest) (this is more accurate than the mid-axillary line)
- Zeroing ensures only the pressure generated within the patient is measured without the interference of external factors. The stopcock closest to the transducer is turned “off to patient” and the cap is removed “open to air” and the monitor’s zero function is activated, then the cap isrpelaced and the stopcock opened to the patient
- The tubing must be the correct type (appropriate compliance and diameter) and length (<4ft) to prevent dynamic response errors (sensitivity to pressure variations)
- Consistency should be used in patient position when obtaining hemodynamic readings, the patient’s backrest can be angled up to 60 degrees in the supine position (any variations in postiion when measured should be documented)
- measurements should be obtained at end expiration for all patients so that there is the least effect of pleural pressure on intracardiac pressures
- See Pulmonary Artery Wedge Pressure and Wedging
Troubleshooting
OTHER INFORMATION
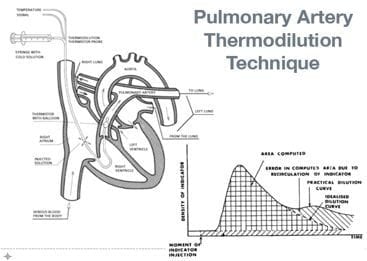
Thermal Dilution
- injection of cold fluid
- recording of change in temperature with time
- rate of blood flow is inversely proportional to the change in temperature over time, according to the Hamilton-Stewart equation
- advantages:
— cheap, non-toxic, arterial puncture avoided, absence of re-circulation - disadvantages:
— requires insertion of PAC, mixing with venous blood may be incomplete, PA blood flow varies greatly with respiration - causes of inaccuracies:
— catheter malposition, injection mistakes (volume, speed, temperature), inaccurate thermistor, TR, intra-cardiac shunts, wrong computation constant
Measured values
- Q: 4-8L/min
- CI: 2.5-4L/min
- CVP: 2-6mmHg
- PAWP: 8-12mmHg
- PAP: 25/10mmHg
- SvO2: 0.65-0.70
- Temperature
Derived values – use of formula: Q = MAP-CVP/SVR
- SV: 50-100mL/beat
- SVI: 25-45mL/beat/m2
- SVR: 900-1300 dynes-sec/cm5
- SVRI: 1900-2400 dyne-sec/cm5
- PVR: 40-150 dyne-sec/cm5
- PVRI: 120-200 dynes-sec/cm5
PAC DATA INTERPRETATION
- Right heart failure: high CVP, low CI, high PVR
- Left heart failure: high PCWP, low CI, high SVR
- Pericardial tamponade: high PCWP, high SVR, CVP = PCWP
- Hypovolemia: low CVP, low PCWP, low CI, high SVR
- Cardiogenic: high CVP, high PCWP, low CI, high SVR
- Sepsis (Distributive): low CVP, low PCWP, high CI, low SVR
PAC COMPLICATIONS
Early
- usual complications of central line insertion
- arrhythmias
- heart block (6% RBBB -> beware if patient already has LBBB!)
- infection
- knotting
- pulmonary infarction
- hypotension
- hypoxia
- PA rupture (see Pulmonary haemorrhage after PAOP measurement)
- air embolism
- valve damage or incompetence
Late
- thrombosis
- pulmonary artery rupture (see Pulmonary haemorrhage after PAOP measurement)
- line sepsis
- endocarditis
- inability to remove (e.g. due to knotting)
PRO/CON DEBATE FOR PAC USE
Arguments for
- in some populations (high risk cardiac surgery) helps to delineate different forms of shock
- may facilitate the early diagnosis of shock (ie. young patients that can maintain MAP in the face of decreasing cardiac output)
- if intra-aortic balloon pump used can still determine cardiac output (unlike PiCCO)
- other monitors also have little data to support their use
- PACMAN study showed they were safe in non-cardiac patients admitted to ICU
Arguments against
- risk of major vessel injury with large bore introducer sheath insertion for PAC
- risk of pulmonary artery haemorrhage
- risk of dysrrhythmia on insertion and/or high grade block if already in LBBB
- data error if PAC not in West Zone III
- we don’t know what an adequate Q is
- absence of data supporting any algorithm to maintain a certain cardiac output
- studies suggest that CVP/RAP and PAOP measurements are inaccurate indicators of RV and LV preload respectively
EVIDENCE SUMMARY
- RCTs have not shown any benefit from PAC use
- early data showed increase harm
- PACMAN trial subsequently showed no change in inhospital mortality, suggesting it can be used safely but gives no benefit
- probably better to use PACs in ‘really sick’ patients
- PAC insertion and use is an invasive procedure with highly morbid complications (e.g. bleeding on insertion, PA rupture, arrhythmia, PE)
- PAC use does have the potential change management in sick patients in the early resuscitation phase
See also Pulmonary Artery Catheter Literature Summaries
AN APPROACH TO PAC USE
- potentially useful in undifferentiated, multi-factorial shock states (for Q and ScVO2)
- useful in right heart pathology and pulmonary hypertension
- requires careful patient selection (including a contraindication assessment)
- don’t wedge (PADP can usually be used to estimate PAOP)
- monitor for complications (predominantly on insertion)
- remove after 72 hours
References and Links
Introduction to ICU Series
Introduction to ICU Series Landing Page
DAY TO DAY ICU: FASTHUG, ICU Ward Round, Clinical Examination, Communication in a Crisis, Documenting the ward round in ICU, Human Factors
AIRWAY: Bag Valve Mask Ventilation, Oropharyngeal Airway, Nasopharyngeal Airway, Endotracheal Tube (ETT), Tracheostomy Tubes
BREATHING: Positive End Expiratory Pressure (PEEP), High Flow Nasal Prongs (HFNP), Intubation and Mechanical Ventilation, Mechanical Ventilation Overview, Non-invasive Ventilation (NIV)
CIRCULATION: Arrhythmias, Atrial Fibrillation, ICU after Cardiac Surgery, Pacing Modes, ECMO, Shock
CNS: Brain Death, Delirium in the ICU, Examination of the Unconscious Patient, External-ventricular Drain (EVD), Sedation in the ICU
GASTROINTESTINAL: Enteral Nutrition vs Parenteral Nutrition, Intolerance to EN, Prokinetics, Stress Ulcer Prophylaxis (SUP), Ileus
GENITOURINARY: Acute Kidney Injury (AKI), CRRT Indications
HAEMATOLOGICAL: Anaemia, Blood Products, Massive Transfusion Protocol (MTP)
INFECTIOUS DISEASE: Antimicrobial Stewardship, Antimicrobial Quick Reference, Central Line Associated Bacterial Infection (CLABSI), Handwashing in ICU, Neutropenic Sepsis, Nosocomial Infections, Sepsis Overview
SPECIAL GROUPS IN ICU: Early Management of the Critically Ill Child, Paediatric Formulas, Paediatric Vital Signs, Pregnancy and ICU, Obesity, Elderly
FLUIDS AND ELECTROLYTES: Albumin vs 0.9% Saline, Assessing Fluid Status, Electrolyte Abnormalities, Hypertonic Saline
PHARMACOLOGY: Drug Infusion Doses, Summary of Vasopressors, Prokinetics, Steroid Conversion, GI Drug Absorption in Critical Illness
PROCEDURES: Arterial line, CVC, Intercostal Catheter (ICC), Intraosseous Needle, Underwater seal drain, Naso- and Orogastric Tubes (NGT/OGT), Rapid Infusion Catheter (RIC)
INVESTIGATIONS: ABG Interpretation, Echo in ICU, CXR in ICU, Routine daily CXR, FBC, TEG/ROTEM, US in Critical Care
ICU MONITORING: NIBP vs Arterial line, Arterial Line Pressure Transduction, Cardiac Output, Central Venous Pressure (CVP), CO2 / Capnography, Pulmonary Artery Catheter (PAC / Swan-Ganz), Pulse Oximeter
LITFL
- CCC – Pulmonary Artery Catheters
- CCC – Pulmonary Artery Wedge Pressure and Wedging
- CCC – Troubleshooting PAC insertion
- CCC – Troubleshooting PAC measurement
- CCC – Pulmonary haemorrhage after PAOP measurement
- CCC – Pulmonary Artery Catheter Literature Summaries
- CCC – History of the Pulmonary Artery Catheter
- DerangedPhysiology.com — Pulmonary Artery Catheter (highly recommended)
- DerangedPhysiology.com — Troubleshooting the insertion of the Pulmonary Artery Catheter
Journal articles
- Hadian M, Pinsky MR. Evidence-based review of the use of the pulmonary artery catheter: impact data and complications. Crit Care. 2006;10 Suppl 3:S8. Review. PubMed PMID: 17164020; PubMed Central PMCID: PMC3226129.
- Payen D, Gayat E. Which general intensive care unit patients can benefit from placement of the pulmonary artery catheter? Crit Care. 2006;10 Suppl 3:S7. Review. PubMed PMID: 17164019; PubMed Central PMCID: PMC3226130.
- Ranucci M. Which cardiac surgical patients can benefit from placement of a pulmonary artery catheter? Crit Care. 2006;10 Suppl 3:S6. Review. PubMed PMID: 17164018; PubMed Central PMCID: PMC3226128.
- Robin E, Costecalde M, Lebuffe G, Vallet B. Clinical relevance of data from the pulmonary artery catheter. Crit Care. 2006;10 Suppl 3:S3. Review. PubMed PMID: 17164015; PubMed Central PMCID: PMC3226125.
- Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970 Aug 27;283(9):447-51. PubMed PMID: 5434111.

Critical Care
Compendium
Chris is an Intensivist and ECMO specialist at The Alfred ICU, where he is Deputy Director (Education). He is a Clinical Adjunct Associate Professor at Monash University, the Lead for the Clinician Educator Incubator programme, and a CICM First Part Examiner.
He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives. He was one of the founders of the FOAM movement (Free Open-Access Medical education) has been recognised for his contributions to education with awards from ANZICS, ANZAHPE, and ACEM.
His one great achievement is being the father of three amazing children.
On Bluesky, he is @precordialthump.bsky.social and on the site that Elon has screwed up, he is @precordialthump.
| INTENSIVE | RAGE | Resuscitology | SMACC
